Products may be shipped at a different temperature than the recommended long-term storage temperature. If the product quality is sensitive to short-term exposure to conditions other than the recommended long-term storage, it will be shipped on wet or dry-ice. If the product quality is NOT affected by short-term exposure to conditions other than the recommended long-term storage, it will be shipped at ambient temperature. As shipping routes are configured for minimum transit times, shipping at ambient temperature helps control shipping costs for our customers. For more information, please refer to the Storage and Transport Conditions document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/316/622/storage-transport-conditions-mk.pdf
445703
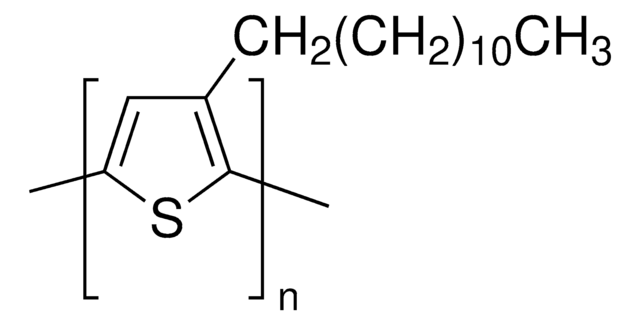
Poly(3-hexylthiophene-2,5-diyl)
regioregular
Sinônimo(s):
P3HT
Selecione um tamanho
Selecione um tamanho
About This Item
Produtos recomendados
Nível de qualidade
peso molecular
average Mw 50,000-100,000
características do produto alternativo mais ecológico
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
condutividade
~103 S/cm (when doped with iodine)
pf
238 °C
238 °C
fluorescência
λex 443 nm; λem 568 nm in chloroform
Energia orbital
HOMO 5 eV
LUMO 3 eV
Desempenho do dispositivo OPV
ITO/NiO/P3HT/PC61BM/LiF/Al
- Short-circuit current density (Jsc): 11.3 mA/cm2
- Open-circuit voltage (Voc): 0.64 V
- Fill Factor (FF): 0.69
- Power Conversion Efficiency (PCE): 5.16 %
ITO/PEDOT:PSS/P3HT:PC61BM (1:08)/Al
- Short-circuit current density (Jsc): 9.5 mA/cm2
- Open-circuit voltage (Voc): 0.63 V
- Fill Factor (FF): 0.68
- Power Conversion Efficiency (PCE): 5 %
categoria alternativa mais ecológica
propriedades semicondutoras
P-type (mobility=1E-4-1E-1 cm2/V·s)
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Aplicação
Características e benefícios
Good processibility, environmental stability and electroactivity.
Embalagem
Informações legais
Rieke is a registered trademark of Rieke Metals, Inc.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Flexible electronic circuits and displays based on organic active materials are future generations of products that may eventually enter mainstream electronics market.
PCBM-based n-type semiconductors - Find p- and n-type organic semiconductors available with PCBM library & properties.
Flexible electronic circuits, displays, and sensors based on organic active materials will enable future generations of electronics products that may eventually enter the mainstream electronics market.
The application of conducting polymers at the interface with biology is an exciting new trend in organic electronics research.
-
How is shipping temperature determined? And how is it related to the product storage temperature?
1 answer-
Helpful?
-
-
How can I determine the shelf life / expiration / retest date of this product?
1 answer-
If this product has an expiration or retest date, it will be shown on the Certificate of Analysis (COA, CofA). If there is no retest or expiration date listed on the product's COA, we do not have suitable stability data to determine a shelf life. For these products, the only date on the COA will be the release date; a retest, expiration, or use-by-date will not be displayed.
For all products, we recommend handling per defined conditions as printed in our product literature and website product descriptions. We recommend that products should be routinely inspected by customers to ensure they perform as expected.
For products without retest or expiration dates, our standard warranty of 1 year from the date of shipment is applicable.
For more information, please refer to the Product Dating Information document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/449/386/product-dating-information-mk.pdfHelpful?
-
-
For Product 445703, Poly(3-hexylthiophene-2,5-diyl), what does regioregular mean?
1 answer-
Regioregular means all of the monomer units are in the same orientation. E.g. if you think of each monomer unit as your palm, regioregular would have all palms facing away, fingers up - all in same orientation. (Regiorandom might have one pointing up, one pointing down, one with palms facing you, one with palms facing away, etc, all in a row.)
Helpful?
-
-
For product 445703, Poly(3-hexylthiophene-2,5-diyl), what are the end groups for the polymer? What is the initiator for the polymerization?
1 answer-
For product 445703, Poly(3-hexylthiophene-2,5-diyl), one end group is a hydrogen and the other end group is a bromine. The name of the initiator for the polymerization is proprietary information.
Helpful?
-
-
For Product 445703, Poly(3-hexylthiophene-2,5-diyl), what does "diyl" refer to?
1 answer-
The use of "diyl" is simply an extension of the use of the "yl" ending for a single substituent. For example, a monosubstituted ethane becomes ethyl - - - -, a monosubstituted propane becomes propyl - - - -, a monosubstituted butane becomes butyl - - - - and so on. Similarly, a 1,3-disubstituted butane becomes 1,3-butanediyl, indicating the presence of similar substituents on the 1- and 3- positions of the butane portion of the molecule.
Helpful?
-
-
What percentage is regioregular in Product 445703, Poly(3-hexylthiophene-2,5-diyl)?
1 answer-
Based on proton NMR, it is estimated to be approximately 98% regioregular.
Helpful?
-
-
What is Product 445703, Poly(3-hexylthiophene-2,5-diyl), soluble in?
1 answer-
This product will be soluble in Carbon Tetrachloride, Chloroform, and Dichloromethane. It is also partially soluble in Tetrahydrofuran, ether and slightly soluble in Toluene.
Helpful?
-
-
What is the Department of Transportation shipping information for this product?
1 answer-
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Helpful?
-
-
What is the molecular weight of Product 445703, Poly(3-hexylthiophene-2,5-diyl)?
1 answer-
The molecular weight (MW) is approximately 87,000.
Helpful?
-
Active Filters
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica

![[6,6]-Phenyl C61 butyric acid methyl ester ≥99%](/deepweb/assets/sigmaaldrich/product/structures/359/221/d990c746-0960-4c69-bf76-fe09b193824d/640/d990c746-0960-4c69-bf76-fe09b193824d.png)