421235
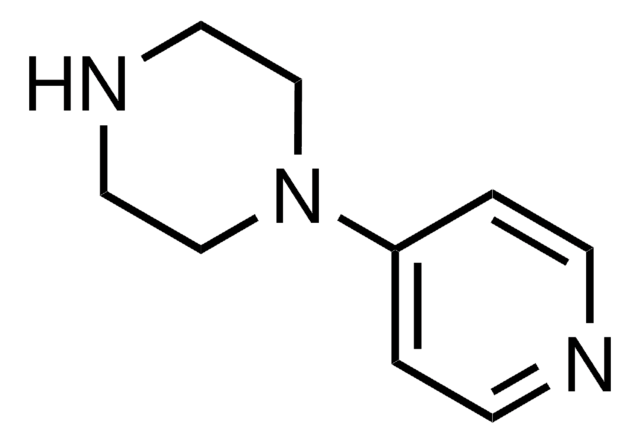
1-(2-Pyrimidyl)piperazine
98%
Sinônimo(s):
2-(1-Piperazinyl)pyrimidine
About This Item
Produtos recomendados
Ensaio
98%
índice de refração
n20/D 1.587 (lit.)
pb
277 °C (lit.)
densidade
1.158 g/mL at 25 °C (lit.)
cadeia de caracteres SMILES
C1CN(CCN1)c2ncccn2
InChI
1S/C8H12N4/c1-2-10-8(11-3-1)12-6-4-9-5-7-12/h1-3,9H,4-7H2
chave InChI
MRBFGEHILMYPTF-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- As derivatization reagent for the carboxyl groups on peptides.
- Carboxy group derivatization during the spectrophotometric analysis of phosphopeptides.
- Starting reagent for the synthesis of 3-{(4-(pyrimidin-2-yl)piperazin-1-yl)methyl}-1H-pyrrolo[2,3-b]pyridine.
- Synthesis of 3-phenyl-6-(4-(pyrimidin-2-yl)piperazin-1-yl)pyridazin-4-ol.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
>230.0 °F
Ponto de fulgor (°C)
> 110 °C
Equipamento de proteção individual
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica