About This Item
Fórmula empírica (Notação de Hill):
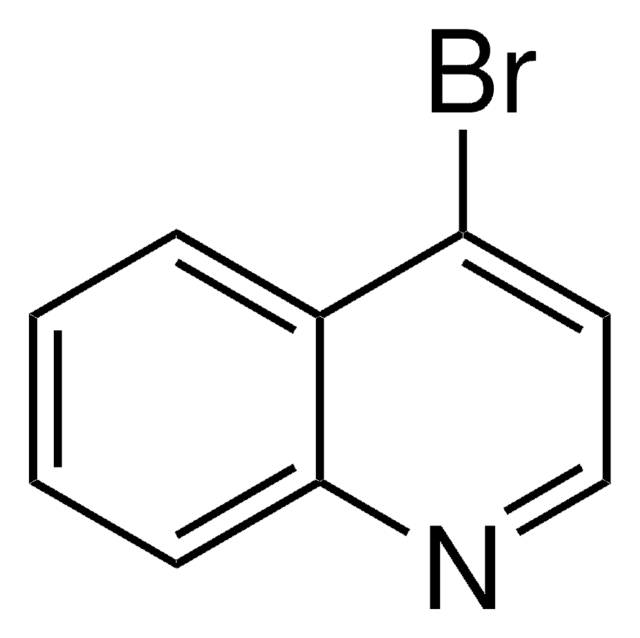
C9H6BrN
Número CAS:
Peso molecular:
208.05
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
98%
índice de refração
n20/D 1.672 (lit.)
p.e.
112-113 °C/0.5 mmHg (lit.)
densidade
1.594 g/mL at 25 °C (lit.)
grupo funcional
bromo
cadeia de caracteres SMILES
Brc1cccc2cccnc12
InChI
1S/C9H6BrN/c10-8-5-1-3-7-4-2-6-11-9(7)8/h1-6H
chave InChI
PIWNKSHCLTZKSZ-UHFFFAOYSA-N
Descrição geral
8-Bromoquinoline is a quinolone derivative. It is widely employed for the synthesis of dyes, food colors, pharmaceutical reagents, pH indicators and in various industrial processes. Its molecule bears a pyridyl group. It undergoes direct heteroarylation reaction with various heteroaromatic compounds in the presence of a palladium catalyst to afford polyheteroaromatic derivatives.
Aplicação
8-Bromoquinoline may be used in the following studies:
- Synthesis of 8-(dimesitylboryl)quinolone (ambiphilic molecule).
- Direct synthesis of 5H-pyrido[3,2,1-ij]quinolin-3-one, via palladium catalyzed coupling reaction with acrolein.
- Preparation of 8-(1-hydroxyethyl)quinolone.
- Preparation of 8-quinolylcyclopentadienyl metal complexes, via reaction with zincated cyclopentadienyl derivatives of Fe, Mn and Re in the presence of bis(triphenylphosphine)palladium(0).
- Synthesis of n,n′-biquinolines by a coupling reaction using tris(triphenylphosphine)nickel(0) and a zerovalent pyridine-nickel complex.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
235.4 °F - closed cup
Ponto de fulgor (°C)
113 °C - closed cup
Equipamento de proteção individual
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Direct heteroarylation of 5-bromothiophen-2-ylpyridine and of 8-bromoquinoline via palladium-catalysed C-H bond activation: simpler access to heteroarylated nitrogen-based derivatives.
Laroche J, et al.
Catalysis Science & Technology, 3(8), 2072-2080 (2013)
Facile synthesis of 8-substituted quinolines.
Suggs JW and Pearson GDN.
The Journal of Organic Chemistry, 45(8), 1514-1515 (1980)
Jung-Ho Son et al.
Dalton transactions (Cambridge, England : 2003), 39(45), 11081-11090 (2010-10-23)
The ambiphilic molecule 8-(dimesitylboryl)quinoline (1) was synthesized by treatment of 8-bromoquinoline or 8-iodoquinoline with n-BuLi followed by dimesitylboronfluoride. Hydrolysis of 1 is unusually rapid compared to bulky triorganoboranes with the sequential loss of mesitylene and formation of mesityl(quinolin-8-yl)borinic acid (2)
Sébastien Noël et al.
Organic & biomolecular chemistry, 4(20), 3760-3762 (2006-10-07)
Unexpectedly, the palladium catalyzed coupling reaction of acrolein with 8-bromoquinoline gave 5H-pyrido[3,2,1-ij]quinolin-3-one in a single step.
Synthesis and coordination behaviour of the new (8-quinolyl) cyclopentadienyl ligand.
Enders M, et al.
Journal of Organometallic Chemistry, 622(1), 66-73 (2001)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica