700023P
Avanti
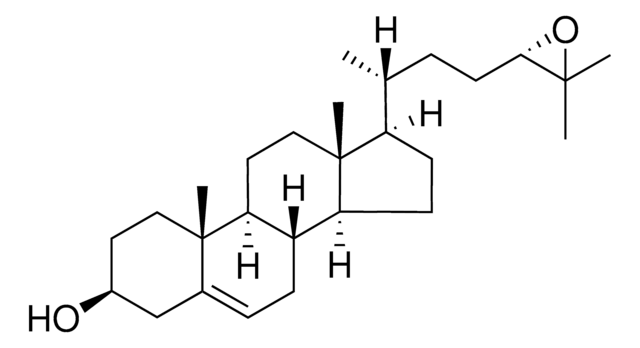
7α,27-dihydroxy-4-cholesten-3-one
Avanti Research™ - A Croda Brand
Synonyme(s) :
Cholest-4-en-3-one, 7,26-dihydroxy-, (7α,25R)-
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C27H44O3
Numéro CAS:
Poids moléculaire :
416.64
Code UNSPSC :
12352211
Nomenclature NACRES :
NA.25
Produits recommandés
Forme
powder
Conditionnement
pkg of 1 × 1 mg (700023P-1mg)
Fabricant/nom de marque
Avanti Research™ - A Croda Brand
Conditions d'expédition
dry ice
Température de stockage
−20°C
Catégories apparentées
Description générale
7α,27-dihydroxy-4-cholesten-3-one synthesized by the hydroxylation of 27-hydroxycholesterol in the presence of the enzyme cholesterol 7 α-hydroxylase (CYP7A1) and is catabolized to bile acid. 7α,27-dihydroxy-4-cholesten-3-one is present majorly in fibroblasts and its conversion from cholesterol occurs in extrahepatic tissues.
Application
7α,27-dihydroxy-4-cholesten-3-one may be used as a ligand to test its effect on Epstein-Barr virus-induced molecule 2 (EB12) activation in guanosine 5′-O-(3-thio)triphosphate ([35S] GTPγS) binding assay.
Actions biochimiques/physiologiques
7α,27-dihydroxy-4-cholesten-3-one is a suppressor of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase.
Conditionnement
5 mL Amber Glass Screw Cap Vial (700023P-1mg)
Informations légales
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Code de la classe de stockage
11 - Combustible Solids
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Hepatic and extrahepatic dehydrogenation/isomerization of 5-cholestene-3beta, 7alpha-diol: localization of 3beta-hydroxy-Delta5-C27-steroid dehydrogenase in pig tissues and subcellular fractions
Furster C
Biochimica et Biophysica Acta - Molecular and Cell Biology of Lipids, 1436(3), 343-353 (1999)
27-Hydroxylated Low Density Lipoprotein (LDL) Cholesterol Can Be Converted to 7alpha, 27-Dihydroxy-4-cholesten-3-one (Cytosterone) before Suppressing Cholesterol Production in Normal Human Fibroblasts EVIDENCE THAT AN ALTERED METABOLISM OF LDL CHOLESTEROL
Axelson M and Larsson O
The Journal of Biological Chemistry, 271(22), 12724-12736 (1996)
Identification of structural motifs critical for epstein-barr virus-induced molecule 2 function and homology modeling of the ligand docking site
Zhang L, et al.
Molecular Pharmacology, 82(6), 1094-1103 (2012)
On the substrate specificity of human CYP27A1: implications for bile acid and cholestanol formation.
Maria Norlin et al.
Journal of lipid research, 44(8), 1515-1522 (2003-06-05)
The mitochondrial sterol 27-hydroxylase (CYP27A1) is required for degradation of the C27-sterol side chain in bile acid biosynthesis. CYP27A1 seems, however, to have roles beyond this, as illustrated by patients with a deficient sterol 27-hydroxylase due to mutations of the
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique