916080
Diethyl-4-cyclohexyl-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
≥95%
Synonyme(s) :
4-Alkyl-1,4-dihydropyridine reagent, DHP reagent for light-mediated His modification
About This Item
Produits recommandés
Essai
≥95%
Forme
powder
Température de stockage
−20°C
InChI
1S/C19H29NO4/c1-5-23-18(21)15-12(3)20-13(4)16(19(22)24-6-2)17(15)14-10-8-7-9-11-14/h14,17,20H,5-11H2,1-4H3
Clé InChI
GERWBKSVDHUVIT-UHFFFAOYSA-N
Application
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Autres remarques
Open-Air Alkylation Reactions in Photoredox-Catalyzed DNA-Encoded Library Synthesis
A photocatalyst-free photo-induced denitroalkylation of ß-nitrostyrenes with 4-alkyl substituted Hantzsch esters at room temperature
Intermolecular Radical Addition to Ketoacids Enabled by Boron Activation
Oxa- and Azabenzonorbornadienes as Electrophilic Partners under Photoredox/Nickel Dual Catalysis
Exploration of a chiral cobalt catalyst for visible-light-induced enantioselective radical conjugate addition
Produit(s) apparenté(s)
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Active Filters
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique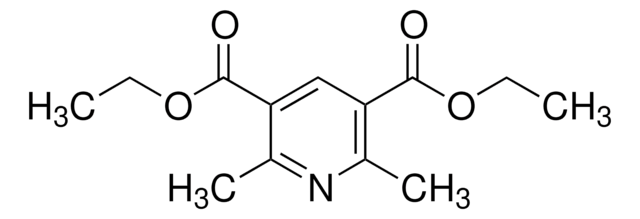






![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)

