329045
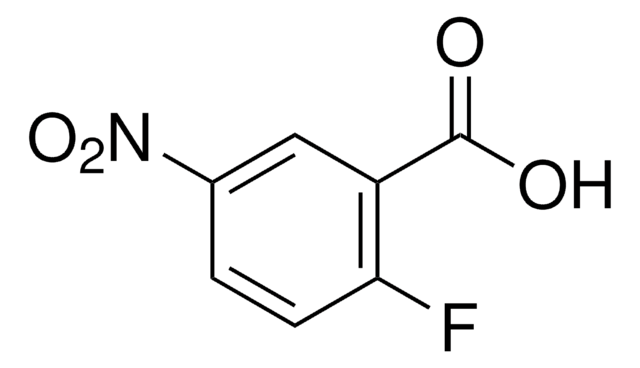
4-Fluoro-3-nitrobenzoic acid
98%
About This Item
Produits recommandés
Pureté
98%
Forme
solid
Pf
123-126 °C (lit.)
Solubilité
95% ethanol: soluble 50 mg/mL, clear, light yellow
Chaîne SMILES
OC(=O)c1ccc(F)c(c1)[N+]([O-])=O
InChI
1S/C7H4FNO4/c8-5-2-1-4(7(10)11)3-6(5)9(12)13/h1-3H,(H,10,11)
Clé InChI
BOJWTAQWPVBIPG-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Application
- as starting reagent in the preparation of novel benzimidazoles having antimycobacterial activity
- in preparation of series of novel acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitors containing benzimidazole core structure
- in preparation of bis(heterocyclic) skeletal precursors for the Pictet-Spengler reaction
- in solid-phase synthesis of trisubstituted [1,3,5]triazino[1,2-a]benzimidazole-2,4(3H,10H)-diones
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique