If this product has an expiration or retest date, it will be shown on the Certificate of Analysis (COA, CofA). If there is no retest or expiration date listed on the product's COA, we do not have suitable stability data to determine a shelf life. For these products, the only date on the COA will be the release date; a retest, expiration, or use-by-date will not be displayed.
For all products, we recommend handling per defined conditions as printed in our product literature and website product descriptions. We recommend that products should be routinely inspected by customers to ensure they perform as expected.
For products without retest or expiration dates, our standard warranty of 1 year from the date of shipment is applicable.
For more information, please refer to the Product Dating Information document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/449/386/product-dating-information-mk.pdf
159220
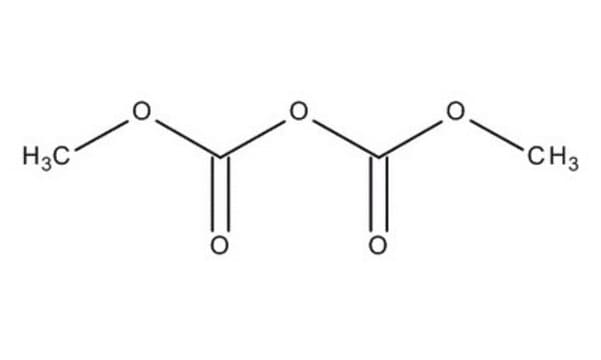
Pyrocarbonate de diéthyle
96% (NT)
Synonyme(s) :
Anhydride éthoxyformique, DEP, DEPC, Dicarbonate de diéthyle, Oxydiformiate de diéthyle
Sélectionner une taille de conditionnement
49,40 €
Sélectionner une taille de conditionnement
About This Item
49,40 €
Produits recommandés
Niveau de qualité
Essai
96% (NT)
Forme
liquid
Indice de réfraction
n20/D 1.398 (lit.)
pb
93-94 °C/18 mmHg (lit.)
Densité
1.101 g/mL at 25 °C (lit.)
Groupe fonctionnel
carbonate
Conditions d'expédition
wet ice
Température de stockage
2-8°C
Chaîne SMILES
CCOC(=O)OC(=O)OCC
InChI
1S/C6H10O5/c1-3-9-5(7)11-6(8)10-4-2/h3-4H2,1-2H3
Clé InChI
FFYPMLJYZAEMQB-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Application
Actions biochimiques/physiologiques
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
156.2 °F - closed cup
Point d'éclair (°C)
69 °C - closed cup
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Solid-state lithium fast-ion conductors are crucial for safer, high-energy-density all-solid-state batteries, addressing conventional battery limitations.
-
How can I determine the shelf life / expiration / retest date of this product?
1 answer-
Helpful?
-
-
How is shipping temperature determined? And how is it related to the product storage temperature?
1 answer-
Products may be shipped at a different temperature than the recommended long-term storage temperature. If the product quality is sensitive to short-term exposure to conditions other than the recommended long-term storage, it will be shipped on wet or dry-ice. If the product quality is NOT affected by short-term exposure to conditions other than the recommended long-term storage, it will be shipped at ambient temperature. As shipping routes are configured for minimum transit times, shipping at ambient temperature helps control shipping costs for our customers. For more information, please refer to the Storage and Transport Conditions document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/316/622/storage-transport-conditions-mk.pdf
Helpful?
-
-
What is the solubility and solution stability of Diethyl pyrocarbonate, DEPC?
1 answer-
DEPC can be solubilized in 95% ethanol, e.g. 1.6 mL in 4 mL ethanol gives a clear, colorless solution. DEPC is soluble in alcohols, esters, ketones and other hydrocarbons, but has limited solubility in water (~0.1%). 6 DEPC hydrolyzes in aqueous solution, to form ethanol and carbon dioxide (CO2). Typically a 0.1% DEPC solution (1 mL of DEPC diluted in water to 1 L) is used to inactivate RNase. The DEPC will not immediately dissolve, as evidenced by the appearance of globules. The mixture should be stirred until the globules disappear. DEPC is sensitive to pH. In phosphate buffer at 25 °C, its half-life is 4 minutes at pH 6, and 9 minutes at pH 7. Hydrolysis is accelerated by Tris buffer,7 where at 25 °C, the half-life of DEPC was reported to be 1.25 minutes at pH 7.5, and 0.37 minutes at pH 8.2. DEPC will decompose in solution when autoclaved. For 0.1% DEPC solutions, autoclaving for 15 minutes per liter should be sufficient.
DEPC is very sensitive to moisture. As such, DEPC is packaged under argon to help reduce exposure to moisture. If DEPC is exposed to even traces of moisture, some hydrolysis occurs. The resulting CO2 is more soluble in DEPC solutions at 2–8 °C than at room temperature. As the product is brought to room temperature before opening, the DEPC can become supersaturated with respect to any dissolved gas. After opening the bottle for the first time and each time thereafter, layer nitrogen or argon gas over the DEPC and store the closed bottle at 2–8 °C for optimal stability. It may be helpful to store the bottle inside a sealed plastic bag with desiccant, but with the bottle cap slightly loose. If the bag inflates at all, this indicates some degree of decomposition with possible pressure build-up. Once opened, the bottle should not be kept for more than 9 months. DEPC decomposes at 155 °C. DEPC is also sensitive to ammonia, which causes decomposition to urethane.Helpful?
-
Active Filters
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique