All Photos(1)
About This Item
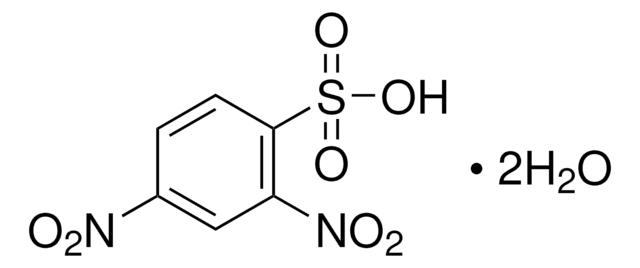
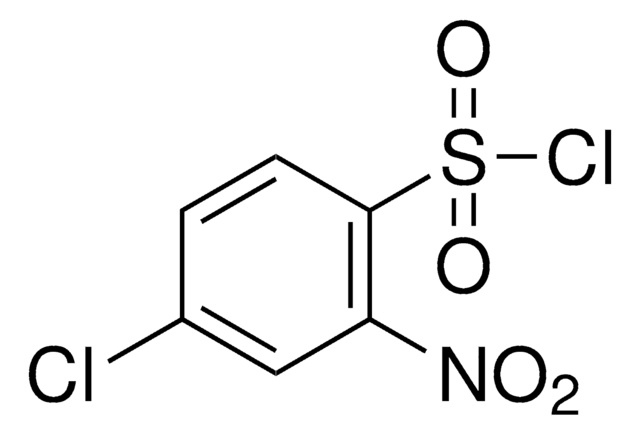
Linear Formula:
(O2N)2C6H3SO2Cl
CAS Number:
Molecular Weight:
266.62
Beilstein:
2147583
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
101-103 °C (lit.)
functional group
nitro
SMILES string
[O-][N+](=O)c1ccc(c(c1)[N+]([O-])=O)S(Cl)(=O)=O
InChI
1S/C6H3ClN2O6S/c7-16(14,15)6-2-1-4(8(10)11)3-5(6)9(12)13/h1-3H
InChI key
SSFSNKZUKDBPIT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,4-Dinitrobenzenesulfonyl chloride causes the sulfonation of glycosylamines to yield N-glycosyl-2,4-dinitrobenzenesulfonamides.
May contain up to 3.5% benzene
Application
2,4-Dinitrobenzenesulfonyl chloride was used to protect primary amines. It was used as starting reagent in the synthesis of tert-butyl 2-[(2,4-dinitrophenyl) sulfonyl]aminoacetate.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 1A - Eye Dam. 1 - Muta. 1B - Skin Corr. 1B - STOT RE 2
Target Organs
Blood
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Rachel J Ball et al.
Artificial DNA, PNA & XNA, 1(1), 27-35 (2011-06-21)
Halogen-labelled peptide organic acid (HPOA) monomers have been synthesised and incorporated into sequence-specific peptide nucleic acid (PNA) probes. Three different types of probe have been prepared; the unmodified PNA probe, the PNA probe with a mass marker, and the PNA
Vishwanath Gaitonde et al.
Journal of carbohydrate chemistry, 31(4-6), 353-370 (2013-01-26)
The N-glycosyl-2,4-dinitrobenzenesulfonamides were accessed via benzoyl-protected β-glycosyl azides. The azides were reduced with Adams' catalyst to the corresponding amines. The glycosylamines were sulfonated with 2,4-dinitrobenzenesulfonyl chloride to form N-glycosyl-2,4-dinitrobenzenesulfonamides in moderate yields. β-Glycosyl amides were then prepared in 67 -
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service