All Photos(1)
About This Item
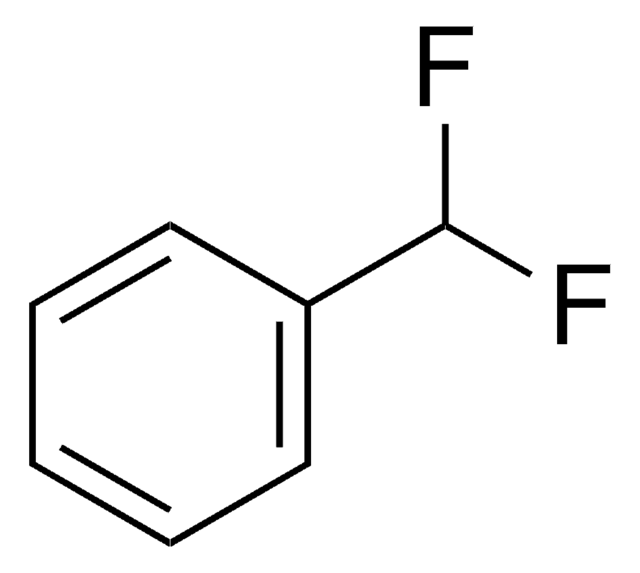
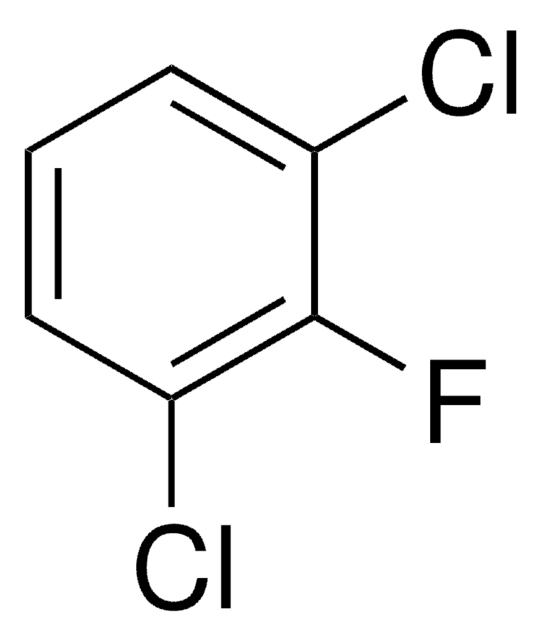
Linear Formula:
CH3C6H3F2
CAS Number:
Molecular Weight:
128.12
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.449 (lit.)
bp
113-117 °C (lit.)
density
1.12 g/mL at 25 °C (lit.)
SMILES string
Cc1ccc(F)cc1F
InChI
1S/C7H6F2/c1-5-2-3-6(8)4-7(5)9/h2-4H,1H3
InChI key
MPXDAIBTYWGBSL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,4-Difluorotoluene was synthesized as a nucleotide analog and was incorporated into DNA and undergoes replication by DNA polymerase enzymes.
Application
2,4-Difluorotoluene was used in the synthesis of new hydrophobic isosteres of pyrimidines and purine nucleosides.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
59.0 °F - closed cup
Flash Point(C)
15 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Thomas W Kirby et al.
Biochemistry, 44(46), 15230-15237 (2005-11-16)
The high fidelity of the DNA polymerization process is critically important for the stability of the cellular genome. The role of template and incoming nucleotide base pairing in polymerase fidelity has recently been explored by the use of nucleotide isosteres
Eric T Kool et al.
Chemical communications (Cambridge, England), (35)(35), 3665-3675 (2006-10-19)
2,4-Difluorotoluene is unusual among hydrofluorocarbons because it is shaped like the DNA base thymine. It was first synthesised as a nucleotide analogue and incorporated into DNA a decade ago. Although it is a nonpolar molecule, it was found to be
M Todd Washington et al.
Molecular and cellular biology, 23(14), 5107-5112 (2003-07-02)
Classical high-fidelity DNA polymerases discriminate between the correct and incorrect nucleotides by using geometric constraints imposed by the tight fit of the active site with the incipient base pair. Consequently, Watson-Crick (W-C) hydrogen bonding between the bases is not required
Danielle A Pfaff et al.
Journal of the American Chemical Society, 130(14), 4869-4878 (2008-03-18)
The incorporation of synthetic nucleoside analogues into DNA duplexes provides a unique opportunity to probe both structure and function of nucleic acids. We used 1H and 19F NMR and molecular dynamics calculations to determine the solution structures of two similar
Angèle Maki et al.
Nucleic acids research, 31(3), 1059-1066 (2003-02-01)
We report the first experimental probing of electrostatic interactions on the pyrimidine side of a bent A tract. Although the curvature of short A tracts (A4-A6) has long been studied, its physical origins remain under debate. Current hypotheses include the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service