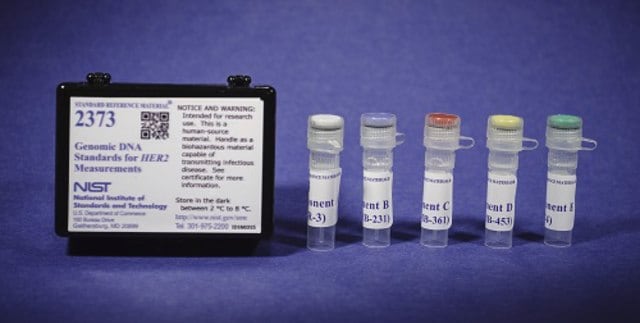
NIST8392
Human DNA for Whole-Genome Variant Assessment (Family Trio of Eastern European Ashkenazi Jewish Ancestry) (HG-002, HG-003, HG-004)
NIST®SRM®
Synonym(e):
Human DNA for Variant Calling (Ashkenazi Jewish) (HG-002, HG-003, HG-004)
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
UNSPSC-Code:
41116107
NACRES:
NA.24
Empfohlene Produkte
Form
liquid
Verpackung
pkg of 10 μg (3 vials)
Hersteller/Markenname
NIST®
Anwendung(en)
genomic analysis
Lagertemp.
−20°C
Allgemeine Beschreibung
Human DNA for Whole-Genome Variant Assessment Reference Material (RM) is intended for validation, optimization, and process evaluation purposes. It consists of three human whole genome samples from a son-father-mother family trio of Eastern European Ashkenazi Jewish ancestry from the Personal Genome Project (IDs huAA53E0, hu6E4515, and hu8E87A9). A unit of RM 8392 consists of three vials containing human genomic DNA from a specific family member; extracted from three large growths of human lymphoblastoid cell lines from the Coriell Institute for Medical Research (Camden, NJ): GM24385 (son) labeled as HG-002, GM24149 (father) labeled as HG-003, and GM24143 (mother) labeled as HG-004. Each vial contains approximately 10 µg of genomic DNA, and the DNA is in TE buffer (10 mM TRIS, 1 mM EDTA, pH 8.0)
SRM 8392_cert
SRM 8392_SDS
SRM 8392_cert
SRM 8392_SDS
Anwendung
Human DNA for Whole-Genome Variant Assessment Reference Material is intended for assessing the performance of human genome sequencing variant calling by obtaining estimates of true positives, false positives, and false negatives. Sequencing applications could include:
- whole genome sequencing
- whole exome sequencing
- targeted sequencing such as gene panels
Leistungsmerkmale und Vorteile
- This reference material contains isolated DNA rather than live cells and is intended for research use.
- An extensive report of the investigation is available through NIST
- Information values are provided for single nucleotide variations (SNVs), small insertions and deletions (indels), and homozygous reference genotypes.
Sonstige Hinweise
- RM 8392 is stored at –20 °C at NIST but will be shipped in freezer packs and may not arrive frozen.
- Details on expiration, storage, safety, usage, and source are provided in the NIST certificate.
- Information on biomaterials, disposal, and transport is available in the SDS.
- he size distributions are measured using PFGE, and biases of this method were not characterized.
Rechtliche Hinweise
NIST is a registered trademark of National Institute of Standards and Technology
SRM is a registered trademark of National Institute of Standards and Technology
Lagerklassenschlüssel
12 - Non Combustible Liquids
WGK
WGK 1
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Choose from one of the most recent versions:
Analysenzertifikate (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Kundensupport
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Determining Performance Metrics for Targeted Next-Generation Sequencing Panels Using Reference Materials
Cleveland MH, et al.
The Journal of Molecular Diagnostics : JMD, 20, 583-590 (2018)
Bennett O V Shum et al.
The Journal of molecular diagnostics : JMD, 19(4), 602-612 (2017-05-16)
The sensitivity and specificity of next-generation sequencing laboratory developed tests (LDTs) are typically determined by an analyte-specific approach. Analyte-specific validations use disease-specific controls to assess an LDT's ability to detect known pathogenic variants. Alternatively, a methods-based approach can be used
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.