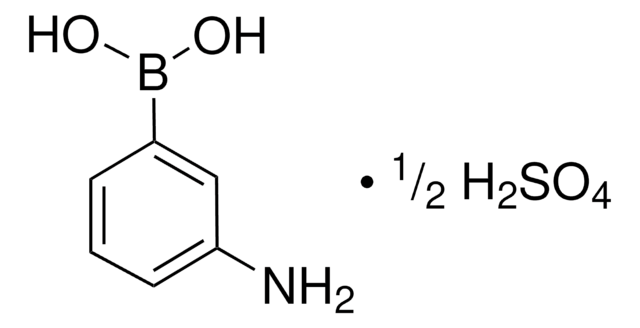
A8312
m-Aminophenylboronic acid–Agarose
aqueous suspension
Sinónimos:
(3-aminophenyl)boronic acid-Agarose, 3-Aminophenylboronic acid–Agarose, m-APBA-Agarose, m-Aminophenylboronic acid resin
About This Item
Productos recomendados
form
aqueous suspension
extent of labeling
40-80 μmol per mL
matrix
6% beaded agarose
matrix activation
epichlorohydrin
matrix attachment
through amino to carboxyls of EDTA
matrix spacer
9 atoms
capacity
≥8 mg/mL binding capacity (peroxidase Type VI)
storage temp.
2-8°C
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
- as a component of the column for the purification of Escherichia coli TOP10F cells
- with cell lysates of various cells for detection of PARylated (Poly(ADP-ribos)ylation) proteins.
- in the affinity purification of horseradish peroxidase (HRPO)
- in the glycated human serum albumin (gHSA) purification
Physical form
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico