8.55019
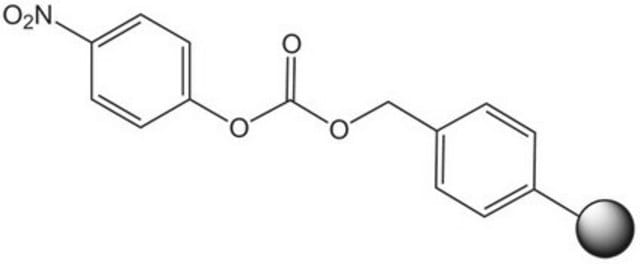
p-Nitrophenyl carbonate Wang resin
Novabiochem®
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
UNSPSC Code:
12352005
Productos recomendados
Quality Level
product line
Novabiochem®
form
beads
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
reactivity: amine reactive
manufacturer/tradename
Novabiochem®
application(s)
peptide synthesis
storage temp.
15-25°C
General description
Resin-bound p-nitrophenyl carbonate esters react readily with amines to provide the corresponding resin-bound carbamate. Leznoff [1] was the first to demonstrate the utility of such resins in solid phase synthesis with the preparation of mono-acylated diamines from symmetrical diamines. Similarly, Dressman, et al. [2] have used carbamate linked resin-bound amino acid amides to make hydantoins via a base-mediated cyclization/cleavage strategy. Amines linked to p-nitrophenyl carbonate Wang resin possess similar chemical properties to methoxybenzyloxycarbonyl protected amines. The resin-bound carbamate is, therefore, cleaved with TFA or hydrogenolysis to afford the free amine [3]. Alternatively, cleavage by reduction with lithium aluminium hydride can be used to generate N-methylamines [4], and Raju & Kogan [5] have utilized this resin to prepare sulfonamides by acylation of immobilized arylamines and cleavage with LiOH or NaOMe. An analogous p-nitrophenyl carbonate resin prepared from NovaSyn TGA resin was employed as a support for the solid phase immobilization of amidines [6]. This resin was also used to synthesize polyamines [7, 8], and to immobilize indoles [9] and guanidinylating reagents [10]. Loading of this resin can be quantified by measuring the release of p-nitrophenolate [11].
Associated Protocols and Technical Articles
Protocols for Loading of Peptide Synthesis Resins
Literature references
[1] D. M. Dixit, et al. (1978) Israel J. Chem., 17, 248.
[2] B. A. Dressman, et al. (1996) Tetrahedron Lett., 37,937.
[3] J. R. Hauske, et al. (1995) Tetrahedron Lett., 36, 1589.
[4] C. Y. Ho & M. J. Kukla (1997) Tetrahedron Lett., 38, 2799.
[5] B. Raju & T. P. Kogan (1997) Tetrahedron Lett., 38, 3373.
[5] A. K. Ghosh, et al. (2001) J. Org. Chem., 66, 2161.
[6] R. Mohan, et al. (1998) Bioorg. Med. Chem. Lett., 8, 1877.
[7] S. Tomasi, et al. (1998) Bioorg. Med. Chem. Lett., 8, 635.
[8] N. D. Hone & L. J. Payne (2000) Tetrahedron Lett., 41, 6149.
[9] A. L. Smith, et al. (2000) Bioorg. Med. Chem. Lett., 10, 2693.
[10] A. K. Ghosh, et al. (2001) J. Org. Chem., 66, 2161.
[11] A. Paio, et al. (2003) Tetrahedron Lett., 44, 1867.
Associated Protocols and Technical Articles
Protocols for Loading of Peptide Synthesis Resins
Literature references
[1] D. M. Dixit, et al. (1978) Israel J. Chem., 17, 248.
[2] B. A. Dressman, et al. (1996) Tetrahedron Lett., 37,937.
[3] J. R. Hauske, et al. (1995) Tetrahedron Lett., 36, 1589.
[4] C. Y. Ho & M. J. Kukla (1997) Tetrahedron Lett., 38, 2799.
[5] B. Raju & T. P. Kogan (1997) Tetrahedron Lett., 38, 3373.
[5] A. K. Ghosh, et al. (2001) J. Org. Chem., 66, 2161.
[6] R. Mohan, et al. (1998) Bioorg. Med. Chem. Lett., 8, 1877.
[7] S. Tomasi, et al. (1998) Bioorg. Med. Chem. Lett., 8, 635.
[8] N. D. Hone & L. J. Payne (2000) Tetrahedron Lett., 41, 6149.
[9] A. L. Smith, et al. (2000) Bioorg. Med. Chem. Lett., 10, 2693.
[10] A. K. Ghosh, et al. (2001) J. Org. Chem., 66, 2161.
[11] A. Paio, et al. (2003) Tetrahedron Lett., 44, 1867.
Linkage
Replaces: 01-64-0123
Analysis Note
Color (visual): white to yellow to beige
Appearance of substance (visual): beads
Loading / photometric determination of p-nitrophenol released upon treatment with piperidine / DMF: 0.60 - 1.20 mmol/g
Swelling Volume (in DMF): lot specific result
The polymer matrix is copoly (styrene - 1% DVB ), 100 - 200 mesh.
Appearance of substance (visual): beads
Loading / photometric determination of p-nitrophenol released upon treatment with piperidine / DMF: 0.60 - 1.20 mmol/g
Swelling Volume (in DMF): lot specific result
The polymer matrix is copoly (styrene - 1% DVB ), 100 - 200 mesh.
Legal Information
Novabiochem is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico