773816
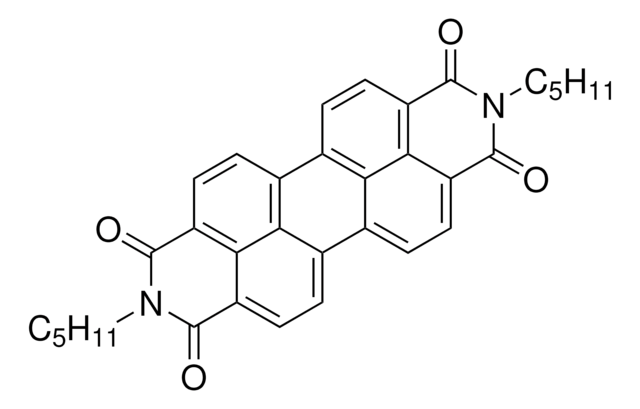
2,9-Dihexylanthra[2,1,9-def:6,5,10-d′e′f′]diisoquinoline-1,3,8,10(2H,9H)tetrone
98%
Sinónimos:
N,N′-Dihexyl-3,4,9,10-perylenedicarboximide, PDI-C6
About This Item
Productos recomendados
assay
98%
form
powder
mp
>360 °C (lit)
λmax
524, 448, 229 nm in dichloromethane
semiconductor properties
N-type (mobility=0.1-2.1 cm2/V·s)
SMILES string
CCCCCCN1C(=O)c2ccc3c4ccc5C(=O)N(CCCCCC)C(=O)c6ccc(c7ccc(C1=O)c2c37)c4c56
InChI
1S/C36H34N2O4/c1-3-5-7-9-19-37-33(39)25-15-11-21-23-13-17-27-32-28(36(42)38(35(27)41)20-10-8-6-4-2)18-14-24(30(23)32)22-12-16-26(34(37)40)31(25)29(21)22/h11-18H,3-10,19-20H2,1-2H3
InChI key
DAMUEXRCHXVMQS-UHFFFAOYSA-N
Application
- High electron transporting character
- Perylenebis(dicarboximide)s (PDIs) can be used as n-type materials for organic fieldeffect transistors (OFETs)
- Good optical properties
- Suitable for use in solution-processed organic phototransistors (OPTs)
- Excellent candidate as an electron accepting building block for organic photovoltaics (OPVs)
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
Intrinsically stretchable active layers for organic field-effect transistors (OFET) are discussed. Polymer structural modification & post-polymerization modifications are 2 methods to achieve this.
Fabrication procedure of organic field effect transistor device using a soluble pentacene precursor.
Solution-processed organic photovoltaic devices (OPVs) have emerged as a promising clean energy generating technology due to their ease of fabrication, potential to enable low-cost manufacturing via printing or coating techniques, and ability to be incorporated onto light weight, flexible substrates.
Thin, lightweight, and flexible electronic devices meet widespread demand for scalable, portable, and robust technology.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico