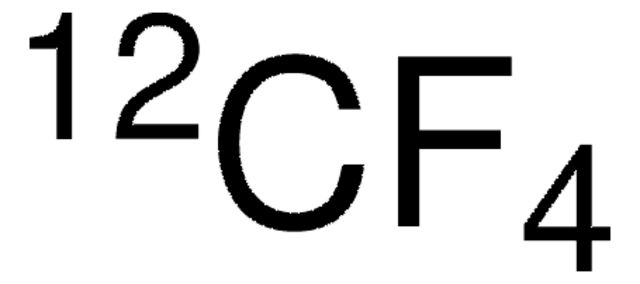
295337
Trifluoromethane
≥98%
Sinónimos:
Fluoroform, HFC-23
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
CHF3
Número de CAS:
Peso molecular:
70.01
EC Number:
MDL number:
UNSPSC Code:
12142100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
vapor density
2.43 (vs air)
vapor pressure
635 psi ( 21 °C)
assay
≥98%
bp
−84 °C (lit.)
mp
−160 °C (lit.)
SMILES string
FC(F)F
InChI
1S/CHF3/c2-1(3)4/h1H
InChI key
XPDWGBQVDMORPB-UHFFFAOYSA-N
Packaging
Supplied in a carbon steel lecture bottle with a CGA180M/CGA110F needle valve installed.
Compatible with the following:
Compatible with the following:
- Aldrich® lecture-bottle station systems
- Aldrich® lecture-bottle gas regulators
Other Notes
See Technical Information Bulletin AL-151 Gas Regulators: Selection, Installation, and Operation
Legal Information
Aldrich is a registered trademark of Sigma-Aldrich Co. LLC
Optional
Referencia del producto
Descripción
Precios
also commonly purchased with this product
Referencia del producto
Descripción
Precios
control valve
hose barb
Referencia del producto
Descripción
Precios
purge valve
Referencia del producto
Descripción
Precios
regulator
signalword
Warning
hcodes
pcodes
Hazard Classifications
Press. Gas Liquefied gas
Storage Class
2A - Gases
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Alessandro Zanardi et al.
Journal of the American Chemical Society, 133(51), 20901-20913 (2011-12-06)
We have found the first reaction of direct cupration of fluoroform, the most attractive CF(3) source for the introduction of the trifluoromethyl group into organic molecules. Treatment of CuX (X = Cl, Br, I) with 2 equiv of MOR (M
Naleen B Jayaratna et al.
Inorganic chemistry, 52(4), 1691-1693 (2013-02-02)
Silver and copper ethylene adducts and the silver carbonyl complex of the tris(pyrazolyl)borate [HB(3,4,5-(CF(3))(3)Pz)(3)](-) (which is based on one of the most acidic pyrazoles known) have been synthesized. (13)C NMR resonance signals of metal-bound ethylene carbon atoms of [HB(3,4,5-(CF(3))(3)Pz)(3)]Ag(C(2)H(4)) and
Mark A Honey et al.
The Journal of organic chemistry, 77(3), 1396-1405 (2012-01-24)
Ethyl 2-diazo-4,4,4-trifluoro-3-oxobutanoate is a highly versatile intermediate for the synthesis of a wide range of trifluoromethyl heterocycles. With the use of rhodium(II) or copper(II) catalyzed carbene X-H insertion reactions as key steps, a diverse set of trifluoromethyl-oxazoles, -thiazoles, -imidazoles, -1,2,4-triazines
Shun Noritake et al.
Organic & biomolecular chemistry, 7(17), 3599-3604 (2009-08-14)
Chiral nonracemic guanidines act as Brønsted bases to generate guanidinium enolates for the enantioselective electrophilic trifluoromethylation of beta-keto esters by means of S-(trifluoromethyl)dibenzothiophenium tetrafluoroborate (Umemoto reagent) with good enantioselectivity of 60-70% range. Despite the fact that the ees are still
Thierry Milcent et al.
Organic & biomolecular chemistry, 8(13), 3025-3030 (2010-05-12)
Trifluoromethyl nitrones were obtained in high yields by condensation of various hydroxylamines with trifluoroacetaldehyde hydrate. The nucleophilic diastereoselective additions of organometallic reagents to these nitrones afforded the corresponding optically active trifluoroethyl hydroxylamines in good yields.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico