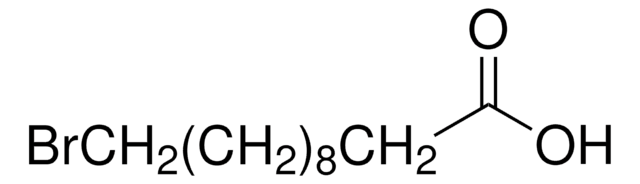All Photos(2)
About This Item
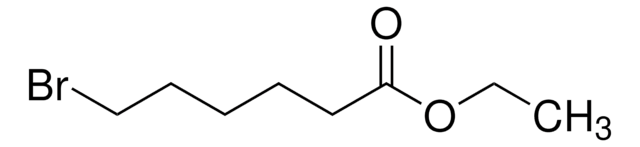
Linear Formula:
Br(CH2)9CO2H
CAS Number:
Molecular Weight:
251.16
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
reaction suitability
reagent type: linker
mp
38-41 °C (lit.)
functional group
bromo
carboxylic acid
SMILES string
OC(CCCCCCCCCBr)=O
InChI
1S/C10H19BrO2/c11-9-7-5-3-1-2-4-6-8-10(12)13/h1-9H2,(H,12,13)
InChI key
PGVRSPIEZYGOAD-UHFFFAOYSA-N
General description
10-Bromodecanoic acid can be prepared from 10-bromodecanol via oxidation.
Application
10-Bromodecanoic acid may be employed as an alkylcarboxylate chain source in the preparation of alkylcarboxylate-grafted polyethylenimine. It may also be used in the synthesis of :
- 10-(methylsulfinyl)decanoic acid
- 10-cyanodecanoic acid
- (10-oxo-10-(4-(3-thioxo-3H-1,2-dithiol-5-yl)phenoxy)-decyl)triphenylphosphonium bromide
- 11-thiastearic acid
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M D Rahman et al.
Journal of medicinal chemistry, 31(8), 1656-1659 (1988-08-01)
A variety of analogues of stearic acid in which one of the methylene groups was replaced by a sulfur atom were examined as inhibitors of growth and fatty acid biosynthesis in the trypanosomatid protozoan Crithidia fasciculata. The 8-, 9-, 10-
Fatemeh Soltani et al.
Artificial cells, nanomedicine, and biotechnology, 45(7), 1356-1362 (2016-11-01)
Poor water solubility of hydrophobic drugs is one of the major problems in pharmaceutical sciences. Herein, in order to address the poor solubility of crocetin, the 30% of primary amines of PAMAM G4 and PPI G4 were alkylated and evaluated
Reza K Oskuee et al.
The journal of gene medicine, 11(10), 921-932 (2009-07-28)
Various strategies have been examined to improve both transfection efficiency and cytotoxicity of polyethylenimine (PEI), a widely used polycationic nonviral gene vector. In the present study, we sought to improve PEI transfection efficiency by combining the osmotic burst mechanism for
Sara Ayatollahi et al.
The international journal of biochemistry & cell biology, 92, 210-217 (2017-10-17)
RNAi-based gene therapy has been recently considered as a promising approach against cancer. Targeted delivery of drug, gene or therapeutic RNAi-based systems to tumor cells is one of the important issues in order to reduce side effects on normal cells.
The synthesis and functional evaluation of a mitochondria-targeted hydrogen sulfide donor,(10-oxo-10-(4-(3-thioxo-3 h-1, 2-dithiol-5-yl) phenoxy) decyl) triphenylphosphonium bromide (Ap39).
Le Trionnaire S, et al.
MedChemComm, 5(6), 728-736 (2014)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service