Tin (Sn)

Tin (Sn) is a silvery-white metal known for its relatively soft, malleable, and ductile characteristics. It is frequently utilized in solder, metals used for bearings, and the production of steel cans used as food containers. Tin exhibits a notable resistance to corrosion due to its ability to form a protective oxide layer when exposed to air.
Read more about
Products

Tin(II) chloride dihydrate
ACS reagent, 98%

Tin(II) chloride
reagent grade, 98%

Tin(IV) chloride pentahydrate
98%

Tin(II) chloride dihydrate
reagent grade, 98%

Sodium (meta)arsenite
≥90%

Dibutyltin dilaurate
95%

Tin(II) 2-ethylhexanoate
92.5-100.0%

Tin(II) iodide
AnhydroBeads™, −10 mesh, 99.99% trace metals basis

Tin(IV) chloride
98%

Tin(II) chloride dihydrate
for analysis EMSURE® ACS,ISO,Reag. Ph Eur

Tin
≥99%, powder

Tributyltin hydride
contains 0.05% BHT as stabilizer, 97%

Tributyltin chloride
96%

Tin(II) chloride dihydrate
puriss. p.a., ACS reagent, reag. ISO, reag. Ph. Eur., ≥98%

Tin(II) fluoride
99%

Tin(IV) oxide
nanopowder, ≤100 nm avg. part. size
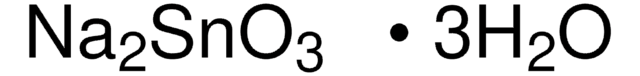
Sodium stannate trihydrate
95%

Tin
powder, <150 μm, 99.5% trace metals basis

Tin(IV) chloride
99.995% trace metals basis

Tin(II) chloride dihydrate
≥99.99% trace metals basis
Tin (Sn) as Catalyst
Compounds containing tin can serve as catalysts, playing a crucial role in expediting chemical reactions. Primarily, they find application in organic synthesis, particularly in the production of fine chemicals and polymers. Catalysts are essential in chemical reactions, enabling the acceleration or modification of reaction rates without undergoing consumption themselves. Tin catalysts can facilitate esterifications and transesterifications, as well as carbon-carbon bond formations. These catalysts prove highly beneficial in organic catalysis and environmental protection due to their notable catalytic activity, ease of manufacture, and reusability. Tin catalysts can be involved in catalytic processes for wastewater treatment and the removal of harmful substances. In addition, tin is employed as a reducing agent in chemical reactions, especially in organic synthesis, facilitating the conversion of functional groups. It exhibits remarkable reducing properties due to its ability to transition between different oxidation states.
Tin Chloride
Tin chloride, also known as stannous chloride with the chemical formula SnCl2, is a white crystalline solid. It serves as a common reducing reagent and finds application in the hydroliquefaction of coals, exhibiting a superior catalytic effect when in a molten state. Tin (II) chloride is also a valuable precursor for semiconducting layers in chemical vapor deposition (CVD). Prepared by reacting its dihydrate form with acetic anhydride, it exhibits reducing properties in acidic media and actively reduces aromatic nitro compounds, nitriles, cyanosilyl ethers, and organic azides. Due to its capacity to produce brighter colors with certain dyes, tin chloride is used as a mordant in textile dyeing. Tin chlorides are also utilized to catalyze the addition of diazo sulfones, diazo phosphine oxides, and diazo phosphonates to aldehydes, forming β-keto phosphine oxides and β-keto phosphonates, respectively. In conjunction with trityl chloride, silyl enol ethers can catalyze the Michael reaction with α,β-unsaturated ketones, and the aldol reaction with acetals or aldehydes.
Tin Iodide
Tin Iodide, also called Stannous Iodide and represented by the chemical formula SnI2, is a solid with a red-orange color. Tin Iodide (SnI2) is used as a precursor material to synthesize lead-free perovskite material for solar cells due to its ability to enhance the stability and performance of the Perovskite solar cells. Tin Iodide helps in controlling the crystallization and growth of the perovskite layer, resulting in improved efficiency and long-term stability of the solar cell. Additionally, it can promote the formation of high-quality perovskite films with good coverage and uniformity, which is crucial for achieving efficient light absorption and charge transport in the solar cell device. Furthermore, high purity Tin Iodide has an excellent property for synthesizing highly stable perovskite thin films for Pb-free light-emitting diodes (PeLEDs). It acts as a dual-functional electrolyte additive in high-rate Li-air batteries. SnI2 protects the lithium anode and reduces charge potential by promoting the decomposition of the Li2O2.
Tin as an Alloy
Tin is renowned for its versatility in forming alloys, a historical influence seen across diverse industries from metallurgy to electronics. An early significant use of tin alloy was in bronze, with compositions of 12.5% tin and 87.5% copper (12.5% and 87.5%, respectively), dating back to 3000 BC. Today, tin is used in a variety of alloys, including widely recognized ones like tin-lead soft solders containing 60% or more tin.
Tin in Electronics
Tin plays an important role in the electronic industry, contributing to the manufacturing and functionality of electronic devices across various applications and formats.
Tin oxide
Tin oxide, chemically represented as SnO, is a metal oxide used in various industries due to its unique properties. It is commonly used in the production of transparent conductive coatings and exhibits both transparency and electrical conductivity when deposited as a thin film. Its broad applications include photovoltaics, optoelectronics as an anode, coating substrate, Schottky diode, and catalyst. Tin (IV) oxide nanopowder belongs to a class of electrode materials applicable in the fabrication of lithium-ion batteries. It offers a high theoretical capacity, good safety, and a low cost of production, which has sparked great interest. Furthermore, surface coating and elemental doping of SnO2 have improved the electrochemical characteristics of batteries. These batteries consist of an anode, cathode, and electrolyte, facilitating a charge-discharge cycle and contributing to the development of greener and sustainable batteries for electrical energy storage. Additionally, the unique characteristics of tin oxide, such as low cost, high gas sensing abilities, low response time, and fast recovery, make it a promising material for gas sensors and optoelectronic devices. Tin oxide is also utilized in the fabrication of mesoporous photoanodes for solar cells. The process involves printing a high-viscosity paste onto semi-processed silica wafers using screen printing, resulting in integrated microarrays with an excellent fabrication yield.
To continue reading please sign in or create an account.
Don't Have An Account?