T90301
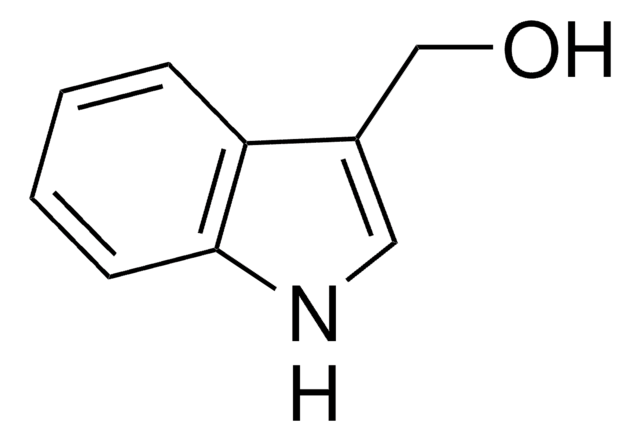
3-(2-Hydroxyethyl)indole
97%
Synonym(s):
2-(3-Indolyl)ethanol, 3-Indoleethanol, IEA, NSC 3884, Tryptophol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H11NO
CAS Number:
Molecular Weight:
161.20
Beilstein:
125553
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
56-59 °C (lit.)
SMILES string
OCCc1c[nH]c2ccccc12
InChI
1S/C10H11NO/c12-6-5-8-7-11-10-4-2-1-3-9(8)10/h1-4,7,11-12H,5-6H2
InChI key
MBBOMCVGYCRMEA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reactant for preparation of:
- Inhibitors of the C-terminal domain of RNA polymerase II and their antitumor activities
- Anti-HIV-1 agents
- Inhibitors of Protein-Protein Interactions
- Partial agonists of the serotonin 5-HT1A receptor
- Growth hormone secretagogues
- Vascular endothelial growth factor (VEGF) inhibitors
- A2B adenosine receptor ligands
- Potential detoxification inhibitors of the crucifer phytoalexin brassinin
- Inhibitors of interleukine 6
- Dual binding site acetylcholinesterase inhibitors
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chuan Liu et al.
Organic letters, 14(17), 4525-4527 (2012-08-16)
Copper(I)-catalyzed dearomative arylation and vinylation of 2-substituted tryptophols were realized with a subsequent cyclization reaction. The cascade dearomatization sequence provided versatile furoindoline derivatives with two quaternary carbon centers in good to excellent yields.
Ivan Kosalec et al.
Arhiv za higijenu rada i toksikologiju, 62(1), 41-49 (2011-03-23)
Tryptophol is an aromatic alcohol and secondary metabolite of the opportunistic fungus Candida albicans. Although its toxicity profile at cell level has been poorly investigated, recent data point to cytotoxic, cytostatic, and genotoxic effects in lymphocytes and the induction of
Olivier Vandeputte et al.
Applied and environmental microbiology, 71(3), 1169-1177 (2005-03-05)
The role and metabolism of indole-3-acetic acid in gram-negative bacteria is well documented, but little is known about indole-3-acetic acid biosynthesis and regulation in gram-positive bacteria. The phytopathogen Rhodococcus fascians, a gram-positive organism, incites diverse developmental alterations, such as leafy
Alessandro Palmieri et al.
Organic & biomolecular chemistry, 10(17), 3486-3493 (2012-03-22)
Reduction of ketosulfonyl indoles with sodium borohydride provides a ready entry to tryptophols in a regiocomplementary fashion with respect to the traditional oxirane ring-opening by indoles under Friedel-Crafts conditions. Compared to traditional β-ketosulfones, ketosulfonyl indoles show a peculiar behavior since
Atiqur Rahman et al.
Bioscience, biotechnology, and biochemistry, 74(11), 2202-2208 (2010-11-13)
Rhizobacteria isolated from wild dipterocarp saplings in Central Kalimantan, Indonesia, were subjected to Salkowski's reagent test, which is often used in detecting indolic substances. Among 69 isolates grown in a low-nitrogen medium supplemented with L-tryptophan (TRP), culture fluids of 29
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service