129453
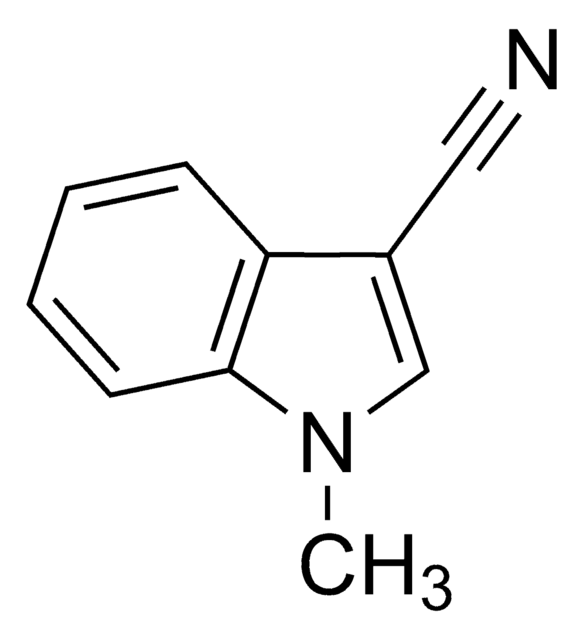
3-Indoleacetonitrile
98%
Synonym(s):
(3-Indolyl)acetonitrile, 3-(Cyanomethyl)indole, IAN, Indolylacetonitrile, NSC 523272
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H8N2
CAS Number:
Molecular Weight:
156.18
Beilstein:
125488
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
bp
157-160 °C/0.2 mmHg (lit.)
mp
33-36 °C (lit.)
functional group
nitrile
SMILES string
N#CCc1c[nH]c2ccccc12
InChI
1S/C10H8N2/c11-6-5-8-7-12-10-4-2-1-3-9(8)10/h1-4,7,12H,5H2
InChI key
DMCPFOBLJMLSNX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3-Indoleacetonitrile (Indolylacetonitrile) is a light-induced auxin-inhibitory substance that is isolated from light-grown cabbage (Brassica olearea L.) shoots. It inhibits the biofilm formation of both E. coli O157:H7 and P. aeruginosa without affecting its growth.
Application
Reactant for preparation of:
- Tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Histone deacetylase inhibitors
- Potential kinase inhibitors
- Kv7/KCNQ potassium channel activators
- Kinesin-Specific MKLP-2 Inhibitor
- Pesticides
- Potential PET cancer imaging agents
- Agonists of the Farnesoid X Receptor (FXR) as atherosclerosis treatment
- Butyrylcholinesterase inhibitors
- Necroptosis inhibitors
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
233.6 °F - closed cup
Flash Point(C)
112 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Light-induced auxin-inhibiting substance from cabbage (Brassica oleacea L.) shoots.
Kosemura S, et al.
Tetrahedron Letters, 38(48), 8327-8330 (1997)
Pedro Robles et al.
Plant physiology, 152(3), 1357-1372 (2010-01-02)
To identify genes involved in vascular patterning in Arabidopsis (Arabidopsis thaliana), we screened for abnormal venation patterns in a large collection of leaf shape mutants isolated in our laboratory. The rotunda1-1 (ron1-1) mutant, initially isolated because of its rounded leaves
Alexandra Kutz et al.
The Plant journal : for cell and molecular biology, 30(1), 95-106 (2002-04-23)
Arabidopsis thaliana expresses four nitrilases, three of which (NIT1, NIT2 and NIT3) are able to convert indole-3-acetonitrile to indole-3-acetic acid (IAA), the plant growth hormone, while the isozyme NIT4 is a beta-cyano-l-alanine hydratase/nitrilase. NIT3 promoter activity is marginal in leaves
Martin de Vos et al.
Plant physiology, 146(3), 916-926 (2008-01-15)
Like many crucifer-specialist herbivores, Pieris rapae uses the presence of glucosinolates as a signal for oviposition and larval feeding. Arabidopsis thaliana glucosinolate-related mutants provide a unique resource for studying the in vivo role of these compounds in affecting P. rapae
Nicole K Clay et al.
Science (New York, N.Y.), 323(5910), 95-101 (2008-12-20)
The perception of pathogen or microbe-associated molecular pattern molecules by plants triggers a basal defense response analogous to animal innate immunity and is defined partly by the deposition of the glucan polymer callose at the cell wall at the site
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service