M28006
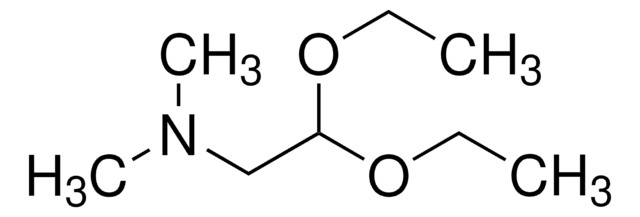
(Methylamino)acetaldehyde dimethyl acetal
97%
Synonym(s):
1,1-Dimethoxy-2-methylaminoethane, 2,2-Dimethoxy-N-methylethylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3NHCH2CH(OCH3)2
CAS Number:
Molecular Weight:
119.16
Beilstein:
605322
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.414 (lit.)
bp
140 °C (lit.)
density
0.928 g/mL at 25 °C (lit.)
SMILES string
CNCC(OC)OC
InChI
1S/C5H13NO2/c1-6-4-5(7-2)8-3/h5-6H,4H2,1-3H3
InChI key
HUMIEJNVCICTPJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
(Methylamino)acetaldehyde dimethyl acetal can be used as a reactant to synthesize:
- Aza[3.3.2] cyclazines by reacting with 5-methyloxazolo[3,2-a]pyridinium salts via synthesis of functionalized 5-aminoindolizines intermediates.
- N-(2,2-Dimethoxyethyl)-N-methyl-3,4-dimethoxyphenylglycine by Petasis reaction with glyoxylic acid and 3,4-dimethoxyphenylboronic acid.
- Substituted imidazoles via copper-catalyzed reaction with various nitriles.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
84.2 °F
Flash Point(C)
29 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Expedient synthesis of substituted imidazoles from nitriles
Frutos RP, et al.
Tetrahedron Letters, 46(48), 8369-8372 (2005)
A concise synthesis of tetrahydroisoquinoline-1-carboxylic acids using a Petasis reaction and Pomeranz-Fritsch-Bobbitt cyclization sequence
Chrzanowska M, et al.
Tetrahedron, 68(14), 3092-3097 (2012)
Rearrangement of oxazolo [3, 2-a] pyridines as an approach of synthesizing aza [3.3. 2] cyclazines
Babaev EV, et al.
Chemistry of Heterocyclic Compounds, 51(3), 269-274 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service