121967
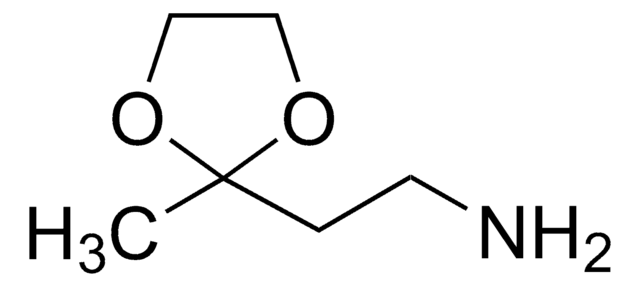
Aminoacetaldehyde dimethyl acetal
99%
Synonym(s):
2,2-Dimethoxyethylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NH2CH2CH(OCH3)2
CAS Number:
Molecular Weight:
105.14
Beilstein:
741868
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
refractive index
n20/D 1.417 (lit.)
bp
135-139 °C/95 mmHg (lit.)
density
0.965 g/mL at 25 °C (lit.)
functional group
acetal
amine
ether
SMILES string
COC(CN)OC
InChI
1S/C4H11NO2/c1-6-4(3-5)7-2/h4H,3,5H2,1-2H3
InChI key
QKWWDTYDYOFRJL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Aminoacetaldehyde dimethyl acetal reacts with sulfone, followed by hydrolysis and reductive amination by adding desired piperazine derivative to yield piperazine derivatives of 2-furanyl[1,2,4]triazolo[1,5-a][1,3,5]triazine.
Aminoacetaldehyde dimethyl acetal is used as a building block for the synthesis of various acylated and sufonylated oxyenamides.
Aminoacetaldehyde dimethyl acetal is used as a building block for the synthesis of various acylated and sufonylated oxyenamides.
Application
Aminoacetaldehyde dimethyl acetal was used in a study to develop a fluorescent substrate for aldehyde dehydrogenase. It was used in preparation of chitosan-dendrimer hybrids having various functional groups such as carboxyl, ester and poly(ethylene glycol). It was used in an efficient 3-step synthesis of a bicyclic proline analog from L-ascorbic acid and in 3-component reaction catalyzed by MgClO4 leading to α-aminophosphonates.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
111.2 °F - closed cup
Flash Point(C)
44 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hitoshi Sashiwa et al.
Biomacromolecules, 4(5), 1244-1249 (2003-09-10)
Chitosan-dendrimer hybrids having various functional groups such as carboxyl, ester, and poly(ethylene glycol) groups were prepared successfully using dendrimer acetal by reductive N-alkylation. The synthetic procedure could be accomplished by one-step reaction without organic solvent. The degree of substitution of
Improved synthesis of imidazole-2-carboxaldehyde, imidazole-2-carboxylic acid, and ethyl imidazole-2-carboxylate
Galeazzi, Eduviges and Guzman
The Journal of Organic Chemistry, 60, 1090-1092 (1995)
R W Storms et al.
Proceedings of the National Academy of Sciences of the United States of America, 96(16), 9118-9123 (1999-08-04)
Because hematopoietic stem cells are rich in aldehyde dehydrogenase (ALDH) activity, we developed a fluorescent substrate for ALDH, termed BODIPY aminoacetaldehyde (BAAA), and tested its potential for isolating primitive human hematopoietic cells. A population of cells with low orthogonal light
Stereoselective Synthesis of 2-Oxyenamides
Krieg, Sara-Cathrin and Grimmer
European Journal of Organic Chemistry (2022)
Chi B Vu et al.
Bioorganic & medicinal chemistry letters, 14(19), 4835-4838 (2004-09-03)
Piperazine derivatives of 2-furanyl[1,2,4]triazolo[1,5-a][1,3,5]triazine have recently been shown to be potent and selective adenosine A(2a) receptor antagonists. We now demonstrate that potent and selective A(2a) receptor antagonists could still be obtained when the arylpiperazines are separated from the triazolotriazine core
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service