753424
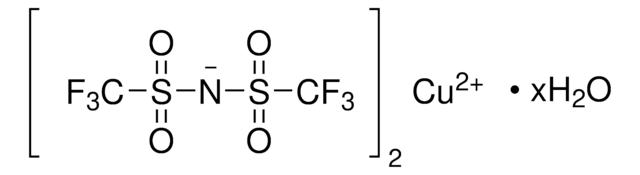
Magnesium bis(trifluoromethanesulfonimide)
Synonym(s):
Magnesium bis(ditriflimide)
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C4F12MgN2O8S4
CAS Number:
Molecular Weight:
584.60
MDL number:
UNSPSC Code:
12352300
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
powder
Quality Level
mp
>200 °C
SMILES string
FC(F)(F)S(=O)(=O)N([Mg]N(S(=O)(=O)C(F)(F)F)S(=O)(=O)C(F)(F)F)S(=O)(=O)C(F)(F)F
InChI
1S/2C2F6NO4S2.Mg/c2*3-1(4,5)14(10,11)9-15(12,13)2(6,7)8;/q2*-1;+2
InChI key
DMFBPGIDUUNBRU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Magnesium bis(trifluoromethanesulfonimide) (Mg(TFSI)2) is a strong magnesium Lewis acid. It is used as a catalyst in organic synthesis. For instance, it is used in the synthesis of dihydropyrazoles by reaction of nitrilimines with alkenes. Additionally, Mg(TFSI)2 salt is also useful for ionic conductivity studies, transference number measurements, and electrochemical properties of gel polymer electrolyte (GPE) systems.
Magnesium bis(trifluoromethanesulfonimide), also known as magnesium triflimide, can be used as an inorganic catalyst in various organic reactions, including acetylation of phenols and alcohols, aminolysis of lactones with amines, [2 + 2] cycloadditions of siloxy-alkynes with carbonyl compounds, cycloisomerization of 1,6-dienes, Friedel-Crafts acylation, and for the synthesis of coumarins.
Application
- Rechargeable Battery Research: Utilized in the study of rechargeable Mg-ion batteries, Magnesium bis(trifluoromethanesulfonimide) serves as a model anode material, providing significant insights into atomistic and mesoscale mechanisms that could revolutionize battery development and application (Kravchyk et al., 2018).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Magnesium bis (trifluoromethane) sulfonimide: An efficient catalyst for the synthesis of coumarins under solvent-free conditions
Wang H
Monatshefte fur Chemie / Chemical Monthly, 144, 411-414 (2013)
Magnesium Bis (trifluoromethylsulfonyl) imide
Selvakumar S, et al.
eEROS (Encyclopedia of Reagents for Organic Synthesis) (2001)
Toshihiko Mandai et al.
Physical chemistry chemical physics : PCCP, 21(23), 12100-12111 (2019-04-26)
To achieve a sustainable-energy society in the future, next-generation highly efficient energy storage technologies, particularly those based on multivalent metal negative electrodes, are urgently required to be developed. Magnesium rechargeable batteries (MRBs) are promising options owing to the many advantageous
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service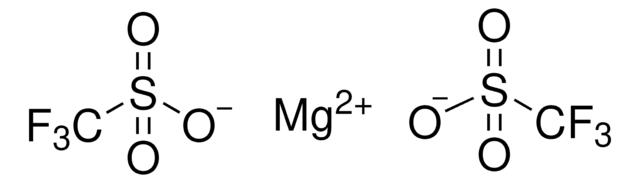



![Zinc di[bis(trifluoromethylsulfonyl)imide] 95%](/deepweb/assets/sigmaaldrich/product/structures/336/073/952daadd-0a7c-4bec-bbaf-442a24c62161/640/952daadd-0a7c-4bec-bbaf-442a24c62161.png)