464635
Trifluoromethanesulfonimide
≥95.0% (19F-NMR)
Synonym(s):
Bis(trifluoromethanesulfonyl)amine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
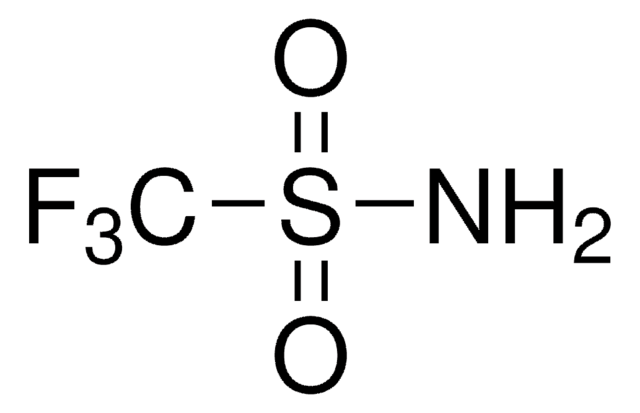
(CF3SO2)2NH
Molecular Weight:
281.15
Beilstein:
4754101
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥95.0% (19F-NMR)
form
solid
bp
90-91 °C (lit.)
mp
46-57 °C (lit.)
functional group
fluoro
SMILES string
FC(F)(F)S(=O)(=O)NS(=O)(=O)C(F)(F)F
InChI
1S/C2HF6NO4S2/c3-1(4,5)14(10,11)9-15(12,13)2(6,7)8/h9H
InChI key
ZXMGHDIOOHOAAE-UHFFFAOYSA-N
Related Categories
General description
Trifluoromethanesulfonimide is a promising Bronsted acid catalyst and is superior to scandium triflate in catalytic action.
Application
Trifluoromethanesulfonimide may be used as a catalyst for the preparation of 1-substituted-1H-1,2,3,4-tetrazoles.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Chronic 3 - Eye Dam. 1
Storage Class Code
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Trifluoromethanesulfonimide catalysed synthesis of 1-substituted-1H-1, 2, 3, 4-tetrazoles using glycerol as green solvent at room temperature.
Wang H, et al.
J. Chem. Res. (M), 40(9), 570-572 (2016)
Practical Synthesis of (?)-a-Tocopherol. Trifluoromethanesulfonimide as an Extremely Active Br?nsted Acid Catalyst for the Condensation of Trimethylhydroquinone with Isophytol.
Ishihara K, et al.
Synlett, 11, 1045-1046 (1996)
Youngbum Kim et al.
Nanoscale, 10(18), 8851-8858 (2018-05-02)
The exciton-dominated light emission of two-dimensional (2D) semiconductors is determined largely by the doping state and the formation of defects. Extensive studies have shown that chemical treatment critically modifies the doping state and defect state of chemical vapor deposition (CVD)-grown
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service