594768
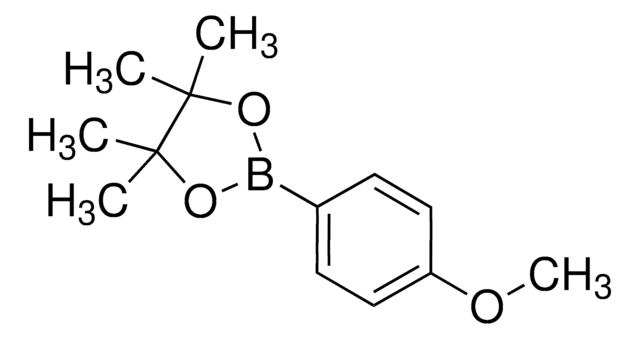
4-Methoxycarbonylphenylboronic acid pinacol ester
97%
Synonym(s):
4-Carbomethoxyphenylboronic acid pinacol ester, Methyl 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzoate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C14H19BO4
CAS Number:
Molecular Weight:
262.11
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
77-81 °C (lit.)
functional group
ester
SMILES string
COC(=O)c1ccc(cc1)B2OC(C)(C)C(C)(C)O2
InChI
1S/C14H19BO4/c1-13(2)14(3,4)19-15(18-13)11-8-6-10(7-9-11)12(16)17-5/h6-9H,1-5H3
InChI key
REIZEQZILPXYKS-UHFFFAOYSA-N
Application
4-Methoxycarbonylphenylboronic acid pinacol ester can be used as a reagent:
- In Suzuki–Miyaura cross-coupling reaction with aryl halides to form C-C bonds.
- For the synthesis of biphenyl derivatives by selective ortho C-H arylation of ketones using Rh catalyst.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of ester-substituted dihydroacridine derivatives and their spectroscopic properties
Suzuki R, et al.
New. J. Chem., 40(3), 2920-2926 (2016)
Kelvin S L Chan et al.
Nature chemistry, 6(2), 146-150 (2014-01-24)
There have been numerous developments in C-H activation reactions in the past decade. Attracted by the ability to functionalize molecules directly at ostensibly unreactive C-H bonds, chemists have discovered reaction conditions that enable reactions of C(sp(2))-H and C(sp(3))-H bonds with
Bing Zhang et al.
Organic letters, 19(21), 5940-5943 (2017-10-20)
A general method for selective ortho C-H arylation of ketone, with boron reagent enabled by rhodium complexes with excellent yields, is developed. The transformation is characterized by the use of air-stable Rh catalyst, high monoarylation selectivity, and excellent yields of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service