All Photos(1)
About This Item
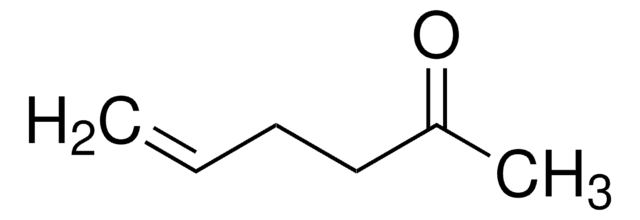
Linear Formula:
(CH3)2CHCH=CHCOCH3
CAS Number:
Molecular Weight:
112.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Assay
75%
form
liquid
impurities
<25% 5-methyl-4-hexen-2-one
refractive index
n20/D 1.44 (lit.)
density
0.85 g/mL at 25 °C (lit.)
functional group
ketone
SMILES string
CC(C)\C=C\C(C)=O
InChI
1S/C7H12O/c1-6(2)4-5-7(3)8/h4-6H,1-3H3/b5-4+
InChI key
IYMKNYVCXUEFJE-SNAWJCMRSA-N
General description
5-methyl-3-hexen-2-one reacts with indole in the presence of pyrrolidine and p-TsOH in CH2Cl2 to yield the 3-substituted indole adduct.
Application
5-Methyl-3-hexen-2-one was used in combinatorial synthesis of mercaptoketones and mercaptoalcohols.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
118.4 °F - closed cup
Flash Point(C)
48 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Dong-Ping Li et al.
Chemical communications (Cambridge, England), (7)(7), 799-801 (2006-02-09)
The use of an equimolar amount of pyrrolidine and HClO4 (30 mol%) was found to be effective in promoting the conjugate addition of indoles to (E)-alpha,beta-unsaturated ketones, affording the corresponding beta-indolyl ketones in excellent yields.
C Vermeulen et al.
Journal of agricultural and food chemistry, 49(11), 5445-5449 (2001-11-21)
Over the past few years, polyfunctional thiols present as trace components have been found to play a major role in many food flavors, due to their exceptionally low odor thresholds. Unfortunately, their presence in minute concentration (in ng/kg to a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service