18265
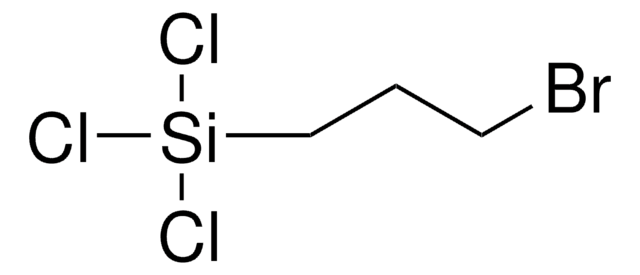
(3-Bromopropyl)trimethoxysilane
≥97.0%
Synonym(s):
γ-Bromopropyltrimethoxysilane, 1-Bromo-3-trimethoxysilylpropane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H15BrO3Si
CAS Number:
Molecular Weight:
243.17
Beilstein:
4952577
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97.0%
form
liquid
refractive index
n20/D 1.440 (lit.)
bp
130 °C/45 mmHg (lit.)
density
1.298 g/mL at 20 °C (lit.)
functional group
bromo
SMILES string
CO[Si](CCCBr)(OC)OC
InChI
1S/C6H15BrO3Si/c1-8-11(9-2,10-3)6-4-5-7/h4-6H2,1-3H3
InChI key
GLISZRPOUBOZDL-UHFFFAOYSA-N
Related Categories
General description
3-bromopropyl trimethoxysilane is a bromo silane. It is popularly used as a silane coupling agent.
Application
3-bromopropyl trimethoxysilane (BPTS) acted as initiating sites for functionalization magnetite (Fe3O4) nanoparticles.{31} A study reports the role of BPTS in attaching a polyelectrolyte to an inorganic alumina substrate during the fabrication of a polymeric humidity sensor.{32}
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
179.6 °F - closed cup
Flash Point(C)
82 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Poly (n-isopropylacrylamide)-based hydrogel coatings on magnetite nanoparticles via atom transfer radical polymerization.
Frimpong RA, et al.
Nanotechnology, 19(17), 175101-175101 (2008)
Polymeric humidity sensor using polyelectrolyte derived from poly (amide-sulfone) s.
Jeon YM, et al.
Macromolecular Research, 17(4), 227-231 (2009)
Qiang Li et al.
Journal of colloid and interface science, 511, 285-295 (2017-10-17)
In this work, N-alkylated poly (4-vinylpyridine) (NPVP), a cationic polymer, was firstly applied for the surface modification of Fe
Servet Tural et al.
Ecotoxicology and environmental safety, 162, 245-252 (2018-07-11)
Click chemistry refers to a group of reactions that are fast, simple to use, easy to purify, versatile, regiospecific, and give high product yields. Therefore, a novel, efficient magnetic nano-sorbent based on N-methyl-D-glucamine attached to magnetic nanoparticles was prepared using
Subodh Kumar et al.
Dalton transactions (Cambridge, England : 2003), 44(26), 11860-11866 (2015-06-10)
Chitosan coated magnetic nanoparticles were synthesized and used as a support for the immobilization of the cobalt(II) acetylacetonate complex [Co(acac)2] and quaternary triphenylphosphonium bromide [P(+)Ph3Br(-)] targeting -NH2 and -OH moieties located on the surface of chitosan. The synthesized material was
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service