A3401
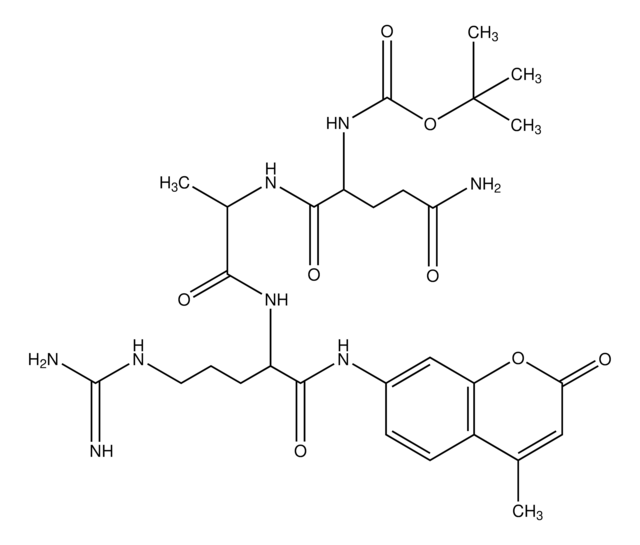
Ala-Ala-Phe-7-amido-4-methylcoumarin
protease substrate
Synonym(s):
H-Ala-Ala-Phe-AMC
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C25H28N4O5
CAS Number:
Molecular Weight:
464.51
MDL number:
UNSPSC Code:
12352204
PubChem Substance ID:
NACRES:
NA.32
Recommended Products
Quality Level
Assay
≥98% (TLC)
form
powder
solubility
ethanol: 20 mg/mL, clear, colorless to light yellow
storage temp.
2-8°C
SMILES string
CC(N)C(=O)NC(C)C(=O)NC(Cc1ccccc1)C(=O)Nc2ccc3C(C)=CC(=O)Oc3c2
InChI
1S/C25H28N4O5/c1-14-11-22(30)34-21-13-18(9-10-19(14)21)28-25(33)20(12-17-7-5-4-6-8-17)29-24(32)16(3)27-23(31)15(2)26/h4-11,13,15-16,20H,12,26H2,1-3H3,(H,27,31)(H,28,33)(H,29,32)
InChI key
FVRLYIFIDKXFHU-UHFFFAOYSA-N
General description
Ala-Ala-Phe-7-amido-4-methylcoumarin is a fluorogenic substrate for the proteolytic activity. It is a positively charged substrate molecule.
Application
Ala-Ala-Phe-7-amido-4-methylcoumarin has been used:
- in the preparation of the reaction mixture for enzymatic assays
- to initiate the enzyme reaction in tripeptidyl peptidase-1 (TPP1) enzyme activity assay
- to initiate the assay reaction in lysosomal hydrolases activity assay
Substrates
Substrate for chymotrypsin and tripeptidyl peptidaseII.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
NADPH oxidase promotes Parkinsonian phenotypes by impairing autophagic flux in an mTORC1-independent fashion in a cellular model of Parkinson?s disease
Pal R, et al.
Scientific reports, 6, 22866-22866 (2016)
Accumulation of polyubiquitylated proteins in response to Ala-Ala-Phe-chloromethylketone is independent of the inhibition of tripeptidyl peptidase II
Villasevil E M, et al.
Biochimica et Biophysica Acta - Molecular Cell Research, 1803(9), 1094-1105 (2010)
Effect of interfacial properties on the activation volume of adsorbed enzymes
Schuabb V, et al.
Colloids and Surfaces. B, Biointerfaces, 140, 497-504 (2016)
R M Bålöw et al.
The Journal of biological chemistry, 261(5), 2409-2417 (1986-02-15)
An extralysosomal tripeptide-releasing aminopeptidase was recently discovered in rat liver (Bålöw, R.-M., Ragnarsson, U., and Zetterqvist, O. (1983) J. Biol. Chem. 258, 11622-11628). In the present work this tripeptidyl peptidase is shown to occur in several rat tissues and in
Raffaella Di Giacopo et al.
Journal of the neurological sciences, 356(1-2), 65-71 (2015-07-06)
This work investigated the molecular cause responsible for a late-onset parkinsonism-dystonia phenotype in three Italian siblings, and clinically characterize this condition. Extensive neurophysiological and neuroradiological exams were performed on the three sibs. Most frequent late-onset metabolic diseases were ruled out
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service