MABN894
Anti-Synapsin-1 Antibody, clone 10.22
clone 10.22, from mouse
Synonym(s):
Synapsin I, Synapsin-1
About This Item
Recommended Products
biological source
mouse
Quality Level
antibody form
purified antibody
antibody product type
primary antibodies
clone
10.22, monoclonal
species reactivity
rat, mouse, human, fish, bovine
technique(s)
immunofluorescence: suitable
immunohistochemistry: suitable (paraffin)
immunoprecipitation (IP): suitable
western blot: suitable
isotype
IgG1
NCBI accession no.
UniProt accession no.
shipped in
wet ice
target post-translational modification
unmodified
Gene Information
human ... SYN1(6853)
General description
Specificity
Immunogen
Application
Western Blotting Analysis: A representative lot detected synapsin Ia/Ib in mouse cortical neuron lysates (Medrihan, L., et al. (2013). Nat. Commun. 4:1512).
Western Blotting Analysis: A representative lot detected purified bovine brain synapsin Ia/Ib (Messa, M., et al. (2010). J. Cell Sci. 123(13):2256-2265).
Western Blotting Analysis: A representative lot detected synapsin-1 in Torpedo (electric ray) synaptosomal preparations and in GST-cyclophilin B pull-downs (Lane-Guermonprez, L., et al. (2005). J. Neurochem. 93(6):1401-1411).
Western Blotting Analysis: A representative lot detected synapsin-1 in the same rat brain subcellular fractions as cyclophilin B (Lane-Guermonprez, L., et al. (2005). J. Neurochem. 93(6):1401-1411).
Western Blotting Analysis: A representative lot detected synapsin Ia/Ib, but not IIa/IIb, in rat brain post-nuclear supernatants (Vaccaro, P., et al. (1997). Brain Res. Mol. Brain Res. 52(1):1-16).
Immunoprecipitation Analysis: A representative lot immunoprecipitated synapsin Ia/Ib, but not IIa/IIb, from rat brain synaptosomal preparations using protein G beads with pre-bound rabbit anti-mouse IgG (Vaccaro, P., et al. (1997). Brain Res. Mol. Brain Res. 52(1):1-16).
Immunofluorescence Analysis: Clone 10.22 ascites fluid was employed to localize synapsin-1 immunoreactivity within retinal inner plexiform layer (IPL) of floating or whole-mount rat retinas (Mandell, J.W., et al. (1992). J. Neurosci. 12(5):1736-1749).
Note: Clone 10.22 does not bind significantly to protein G. For immunoprecipitation application, pre-coat protein G beads with an anti-mouse IgG antibody is recommended (Vaccaro, P., et al. (1997). Brain Res. Mol. Brain Res. 52(1):1-16).
Neuroscience
Developmental Neuroscience
Quality
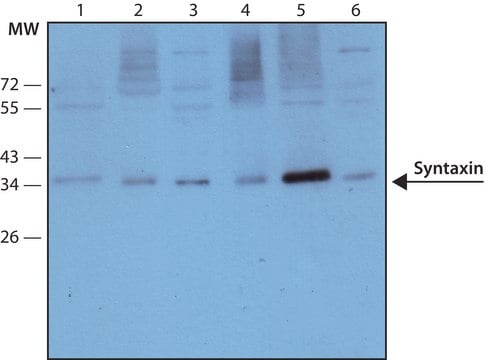
Western Blotting Analysis: 0.5 µg/mL of this antibody detected Synapsin-1 in 10 µg of rat brain cytosol tissue lysate.
Target description
Physical form
Storage and Stability
Other Notes
Disclaimer
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service