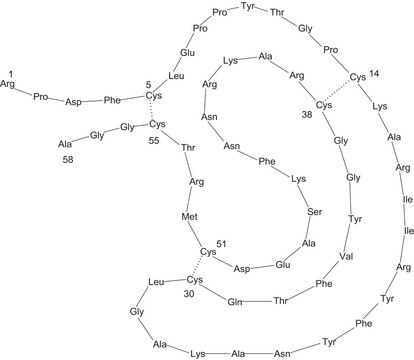
The sequence has been published. The amino acid composition is:Asp5l, Asn45, Thr35, Ser29, Glu64, Gln31, Pro36, Gly48, Ala63, Cys9, Val62, Met2l, Ile49, Leu79, Tyr36, Phe38, Trp12, Lys48, HiS22, and Arg63. Reference for the sequence and amino acid composition: K. Titani et al., Proc. Nat. Acad. Sci., USA, 74, 4762-4766 (1977) (see attached pdf).Product P1261 is phosphorylase a, which is the tetrameric form. Products P4649 and P6635 are phosphorylase b, the dimeric form. The sequence information of the subunit would be the same for both forms.
P6635
Phosphorylase b from rabbit muscle
lyophilized powder, ≥20 units/mg protein, 2× crystallization
Synonym(s):
α-Glucan Phosphorylase, 1,4-α-D-Glucan:orthophosphate α-D-glucosyltransferase, Glycogen Phosphorylase
About This Item
Recommended Products
biological source
rabbit muscle
form
lyophilized powder
specific activity
≥20 units/mg protein
mol wt
97,200 Da by calculation
purified by
2× crystallization
storage condition
(Keep container tightly closed in a dry and well-ventilated place)
technique(s)
mass spectrometry (MS): suitable
impurities
~0.01 μmol/mg protein 5′-AMP (This low level will not interfere with phosphorylase and phosphorylase kinase assays.)
UniProt accession no.
foreign activity
phosphoglucomutase ≤1.0%
phosphorylase a ≤10%
phosphorylase kinase ≤0.5%
phosphorylase phosphatase, debrancher enzyme, AMPase and ATPase ≤0.1%
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
General description
Application
- in the calibration of Sepharose C1-6B columns while studying the molecular weight of methylamine dehydrogenase subunits[3]
- in ion mobility-mass spectrometry studies of phosphorylase B ions that have been generated with supercharging reagents, in the charge-reducing buffer[4]
- for the preparation of p32 labeled phosphorylase A using phosphorylase kinase and [32P]ATP[5]
- in phosphorylase phosphatase assay[6]
- in enzyme assay as a positive control to ensure the reaction system for the activity determination was adopted[7]
Biochem/physiol Actions
Packaging
Unit Definition
Physical form
antibody
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
-
Has the amino acid sequence of phosphorylase from rabbit muscle been published?
1 answer-
Helpful?
-
-
What is the Department of Transportation shipping information for this product?
1 answer-
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Helpful?
-
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service