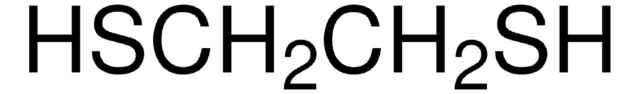T28002
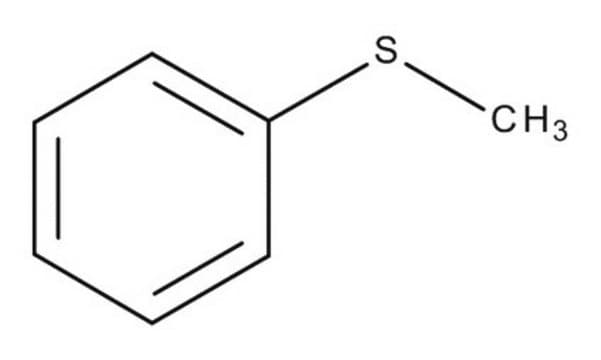
Thioanisole
ReagentPlus®, ≥99%
Synonym(s):
Methyl phenyl sulfide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5SCH3
CAS Number:
Molecular Weight:
124.20
Beilstein:
1904179
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
≥99%
refractive index
n20/D 1.587 (lit.)
bp
188 °C (lit.)
mp
−15 °C (lit.)
density
1.057 g/mL at 20 °C (lit.)
SMILES string
CSc1ccccc1
InChI
1S/C7H8S/c1-8-7-5-3-2-4-6-7/h2-6H,1H3
InChI key
HNKJADCVZUBCPG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
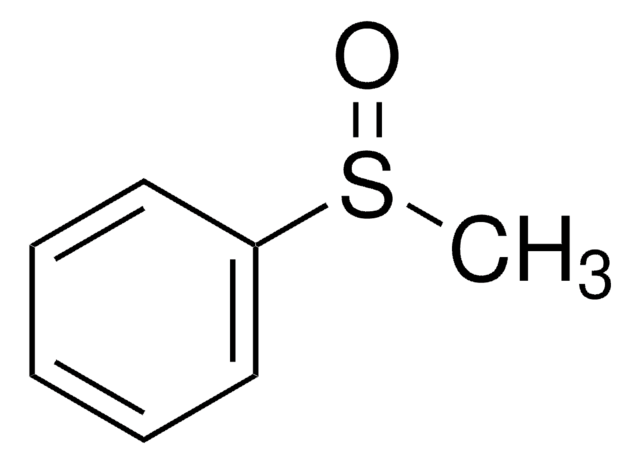
Thioanisole may be used in the synthesis of methyl phenyl sulfoxide via oxidation.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1B
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
163.4 °F - closed cup
Flash Point(C)
73 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mild and selective oxidation of sulfur compounds in trifluoroethanol: diphenyl disulfide and methyl phenyl sulfoxide.
Ravikumar KS, et al.
Organic Syntheses, 184-189 (2003)
Methyl phenyl sulfoxide.
Johnson CR & Keiser JE.
Organic Syntheses, 78-78 (1966)
Wen Wang et al.
Journal of the American Chemical Society, 131(36), 12892-12893 (2009-08-26)
Magnetic nanoparticles (MNPs) with a core diameter of 30 nm comprising several iron oxide crystals, a poly(glycidyl methacrylate) (PGMA) shell with a thickness of 30 nm, and a surface coated with chloroperoxidase (CPO) were facilely fabricated as a nanobiocatalyst for
Rémy Ricoux et al.
Organic & biomolecular chemistry, 7(16), 3208-3211 (2009-07-31)
Two new artificial hemoproteins or "hemozymes", obtained by non covalent insertion of Fe(III)-meso-tetra-p-carboxy- and -p-sulfonato-phenylporphyrin into xylanase A from Streptomyces lividans, were characterized by UV-visible spectroscopy and molecular modeling studies, and were found to catalyze the chemo- and stereoselective oxidation
Jiyun Park et al.
Journal of the American Chemical Society, 133(14), 5236-5239 (2011-03-18)
The mechanism of sulfoxidation of thioaniosoles by a non-heme iron(IV)-oxo complex is switched from direct oxygen transfer to metal ion-coupled electron transfer by the presence of Sc(3+). The switch in the sulfoxidation mechanism is dependent on the one-electron oxidation potentials
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service