All Photos(1)
About This Item
Empirical Formula (Hill Notation):
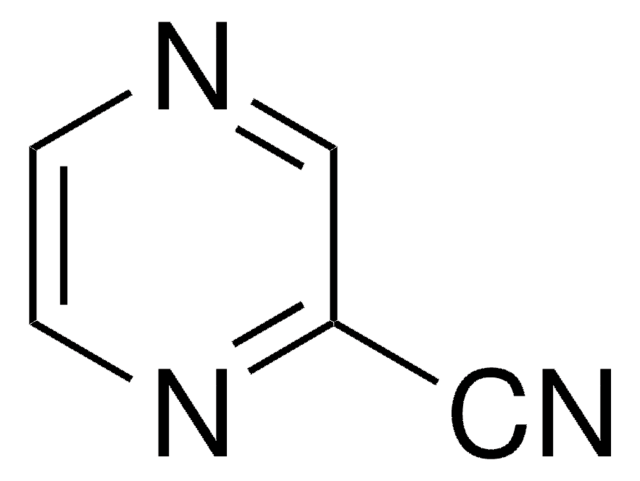
C4H5N3
CAS Number:
Molecular Weight:
95.10
Beilstein:
107025
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
crystals
mp
118-120 °C (lit.)
SMILES string
Nc1cnccn1
InChI
1S/C4H5N3/c5-4-3-6-1-2-7-4/h1-3H,(H2,5,7)
InChI key
XFTQRUTUGRCSGO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Substrate in a four-component synthesis of imidazolidines.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J F Cavalier et al.
Bioorganic & medicinal chemistry, 9(4), 1037-1044 (2001-05-17)
A series of 5-aryl- and 3,5-bis-aryl-2-amino-1,4-pyrazine derivatives 4 and 6, and related imidazolopyrazinones 7, has been synthesized, the aryl groups of which are catechol and/or phenol substituents. These compounds, tested against human keratinocyte cells stressed by UVB irradiation, showed high
W H Lunn et al.
Xenobiotica; the fate of foreign compounds in biological systems, 22(11), 1239-1241 (1992-11-01)
1. The compound 2-aminopyrazine was given by oral gavage to normal rats and their urine collected. 2. A mercapturic acid containing the 2-aminopyrazine moiety was isolated from this urine. This represents the first example of this type of a metabolite
Synthetic Communications, 37, 247-247 (2007)
Anna Eriksson et al.
Biochemical pharmacology, 80(10), 1507-1516 (2010-08-14)
Aberrant signal transduction by mutant or overexpressed protein kinases has emerged as a promising target for treatment of acute myeloid leukemia (AML). We here present a novel low molecular weight kinase inhibitor, AKN-032, targeting the FMS-like tyrosine kinase 3 (FLT3)
Frédéric De Wael et al.
European journal of medicinal chemistry, 45(9), 3564-3574 (2010-06-24)
Based on the imidazo-[1,2-a]-pyrazin-3-(7H)-one scaffold, a dual action prodrug has been designed for combining antioxidant and anti-inflammatory activities, possibly unmasked upon oxidation. The construction of the target-molecule requires two building blocks, namely a 2-amino-1,4-pyrazine and a 2-ketoaldehyde. Attempts to synthesize
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service