B5002
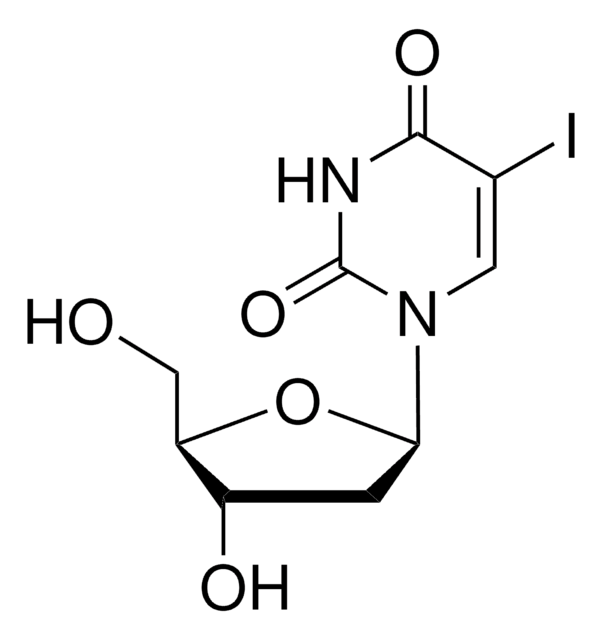
5-Bromo-2′-deoxyuridine
≥99% (HPLC)
Synonym(s):
5-BrdU, 5-Bromo-1-(2-deoxy-β-D-ribofuranosyl)uracil, 5-Bromouracil deoxyriboside, BUdR
About This Item
Recommended Products
biological source
synthetic (organic)
assay
≥99% (HPLC)
form
powder
mp
191-194 °C (dec.) (lit.)
solubility
DMSO: 50 mg/mL, clear, colorless to very faintly yellow
storage temp.
−20°C
SMILES string
OC[C@H]1O[C@H](C[C@@H]1O)N2C=C(Br)C(=O)NC2=O
InChI
1S/C9H11BrN2O5/c10-4-2-12(9(16)11-8(4)15)7-1-5(14)6(3-13)17-7/h2,5-7,13-14H,1,3H2,(H,11,15,16)/t5-,6+,7+/m0/s1
InChI key
WOVKYSAHUYNSMH-RRKCRQDMSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- as a supplement to study the effect of electroconvulsive seizures on hippocampal neurogenesis
- as a supplement to study the loss-of-gene function in adult zebrafish heart
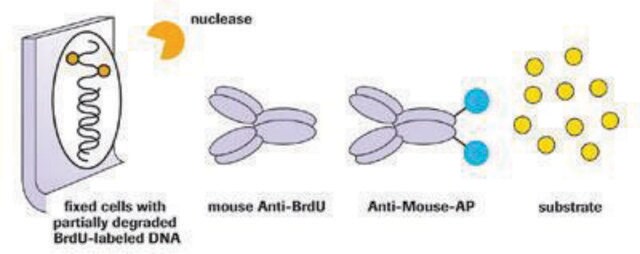
- in T lymphocyte proliferation assay{133]
Biochem/physiol Actions
also commonly purchased with this product
signalword
Danger
hcodes
Hazard Classifications
Muta. 1B - Repr. 2
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 2
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Cell cycle regulates vital processes like DNA repair, cancer prevention. Four stages: G1, S, G2, M. NTPs don't permeate membranes.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service