R-006
Risperidone solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Recommended Products
grade
certified reference material
Quality Level
form
liquid
feature
Snap-N-Spike®/Snap-N-Shoot®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
concentration
1.0 mg/mL in methanol
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
clinical testing
format
single component solution
storage temp.
−20°C
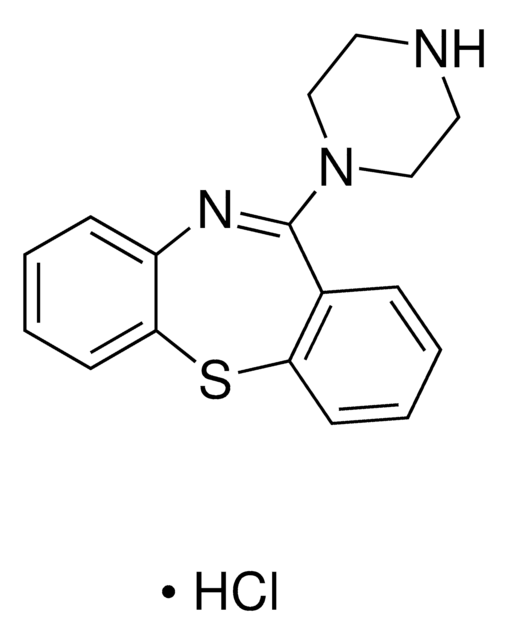
SMILES string
CC1=C(CCN2CCC(CC2)c3noc4cc(F)ccc34)C(=O)N5CCCCC5=N1
InChI
1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3
InChI key
RAPZEAPATHNIPO-UHFFFAOYSA-N
Gene Information
human ... DRD2(1813) , HTR2A(3356) , HTR2C(3358)
General description
Application
- Vascular Syndromes and Neuroleptic Therapy: RisperiDonesolution has been investigated for its role in inducing severe vascular occlusion-like syndromes in rat models. Research highlights its potential implications for understanding drug-induced vascular problems and testing therapies like BPC 157, a gastric pentadecapeptide with promising therapeutic effects (Strbe et al., 2023).
- UHPLC Method Development: RisperiDoneis a focal point in the development of an advanced UHPLC method using the "Method Operable Design Region" (MODR) approach. This method is designed for the assay and purity determination of risperiDonein various formulations, crucial for maintaining stringent quality controls in pharmaceutical manufacturing (Pawar et al., 2022).
- Optimizing Antipsychotic Dose Regimens: Research incorporates risperiDoneto explore Maximum A Posteriori (MAP) Bayesian modelling, merging drug plasma concentrations and dopamine receptor occupancy. This approach aims to customize antipsychotic dosing to enhance therapeutic outcomes in individual patients, emphasizing personalized medicine in psychiatry (Ismail et al., 2022).
- Antioxidant-mediated Drug Degradation: Studies on risperiDonealso cover the formulation aspects, such as its inclusion in poly(ethylene carbonate) systems to control drug degradation through antioxidant mediation. This research is pivotal in developing sustained-release formulations that enhance drug stability and efficacy (Bohr et al., 2020).
- Patient-reported Outcomes in Schizophrenia Treatment: RisperiDonesolution is studied for its effectiveness in treating schizophrenia, particularly assessing patient-reported outcomes in long-term therapy settings. This research helps gauge patient satisfaction and treatment efficacy, which is essential for optimizing mental health therapies (Dhanda et al., 2019).
Legal Information
related product
signalword
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
target_organs
Eyes,Central nervous system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
49.5 °F - closed cup
flash_point_c
9.7 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service