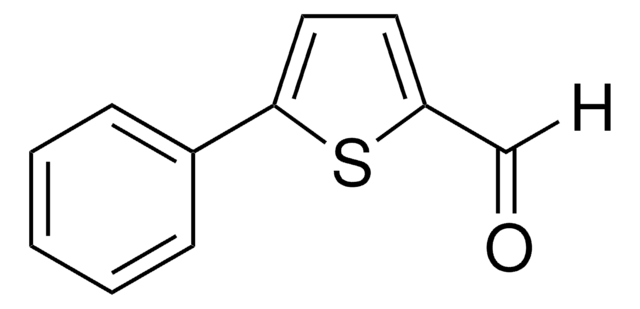
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H6OS2
CAS Number:
Molecular Weight:
194.27
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
mp
55-58 °C (lit.)
SMILES string
[H]C(=O)c1ccc(s1)-c2cccs2
InChI
1S/C9H6OS2/c10-6-7-3-4-9(12-7)8-2-1-5-11-8/h1-6H
InChI key
FYBWRAXKYXTOQC-UHFFFAOYSA-N
Application
2,2′-Bithiophene-5-carboxaldehyde may be used in the synthesis of the following:
- boron dipyrromethene(BODIPY)oligothiophenes via a multi-step reaction process
- (2,2′-bithiophene-5-carbaldehyde)-4-nitrophenylhydrazone(BT-NPH) via reaction with 4-nitrophenylhydrazine
- bithiophene fulleropyrrolidine obtained via refluxing with sarcosine and fullerene
- azomethine phthalic diimides by heating with N,N-bis(4-amino-2,3,5,6-tetramethylphenyl)naphthalene-1,4,5,8-dicarboximide (DANDI)
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
"Optical nonlinearities and molecular conformations in thiophene-based hydrazone crystals"
Kwon P-O, et al.
The Journal of Physical Chemistry C, 113(34), 15405-15411 (2009)
"The effect of thiophene substituents of fulleropyrrolidine acceptors on the performance of inverted organic solar cells"
Kaunisto.MK, et al.
Synthetic Metals, 195, 193-200 (2014)
"New low band gap compounds comprised of naphthalene diimide and imine units"
Schab-Balcerzak E, et al.
Synthetic Metals, 162(05), 543- 553 (2012)
"Enhanced Functionality for Donor?Acceptor Oligothiophenes by means of Inclusion of Bodipy: Synthesis, Electrochemistry, Photophysics, and Model Chemistry"
Collado D, et al.
Chemistry?A European Journal , 17(02), 498-507 (2011)
Sara S M Fernandes et al.
ACS omega, 3(10), 12893-12904 (2018-11-10)
A series of push-pull heterocyclic N,N-diphenylhydrazones were prepared to study the effect of structural modifications (different π-spacers and electron-withdrawing groups) on the optical (linear and nonlinear) and electronic properties of the molecules. The photovoltaic response of dye-sensitized solar cells assembled
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![Benzo[b]thiophene-2-carboxaldehyde 97%](/deepweb/assets/sigmaaldrich/product/structures/321/060/32405a4e-5720-4c6d-91cf-115c747270c4/640/32405a4e-5720-4c6d-91cf-115c747270c4.png)

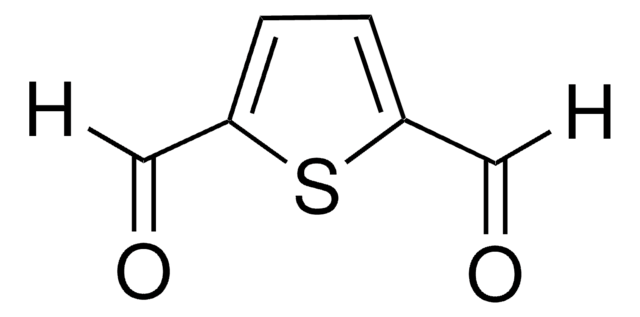
![Thieno[3,2-b]thiophene-2,5-dicarboxaldehyde 96%](/deepweb/assets/sigmaaldrich/product/structures/137/771/57dfbc98-f02d-4773-bc11-3e8b861ad74b/640/57dfbc98-f02d-4773-bc11-3e8b861ad74b.png)