All Photos(1)
About This Item
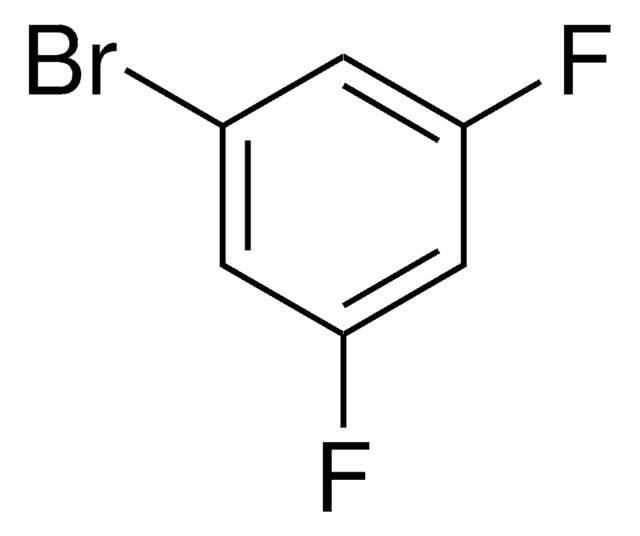
Linear Formula:
F2C6H3NH2
CAS Number:
Molecular Weight:
129.11
Beilstein/REAXYS Number:
2802286
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
form
solid
mp
37-41 °C (lit.)
functional group
fluoro
SMILES string
Nc1cc(F)cc(F)c1
InChI
1S/C6H5F2N/c7-4-1-5(8)3-6(9)2-4/h1-3H,9H2
InChI key
KQOIBXZRCYFZSO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
In vitro nephrotoxic effects of 3,5-difluoroaniline has been investigated in renal cortical slices obtained from the kidneys of untreated, male Fischer 344 rats.
Application
3,5-Difluoroaniline has been used in the preparation of 3,5-difluorodimethylaniline.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
167.0 °F - closed cup
flash_point_c
75 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Further studies on the carcinogenicity of dyes related to 4-dimethylaminoazobenzene; the requirement for an unsubstituted 2-position.
J A MILLER et al.
Cancer research, 17(5), 387-398 (1957-06-01)
S K Hong et al.
Toxicology letters, 114(1-3), 125-133 (2000-03-14)
Haloanilines are widely used as chemical intermediates in the manufacture of pesticides, dyes and drugs. The purpose of this study was to examine the in vitro nephrotoxic effects of the four 4-haloaniline and four 3,5-dihaloaniline isomers using renal cortical slices
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service