All Photos(1)
About This Item
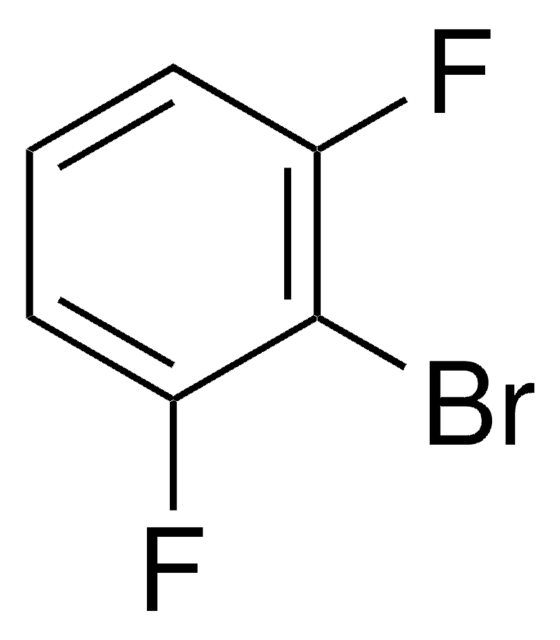
Linear Formula:
BrC6H3F2
CAS Number:
Molecular Weight:
192.99
Beilstein/REAXYS Number:
1680892
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
refractive index
n20/D 1.505 (lit.)
bp
145-146 °C (lit.)
mp
−4 °C (lit.)
density
1.708 g/mL at 25 °C (lit.)
functional group
bromo
fluoro
SMILES string
Fc1ccc(Br)c(F)c1
InChI
1S/C6H3BrF2/c7-5-2-1-4(8)3-6(5)9/h1-3H
InChI key
MGHBDQZXPCTTIH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1-Bromo-2,4-difluorobenzene undergoes lithiation exclusively at the position having two adjacent halogen substituents, to yield 6-bromo-2,3-difluorobenzoic acid.
Application
1-Bromo-2,4-difluorobenzene has been used in:
- preparation of (2S)-1-(2,4-difluorophenyl)-2-(1,1-dimethyl-1-sila-ethoxy)-propan-1-one
- enantiomeric preparation of the key intermediate of chiral azole antifungal agents by a chemoenzymatic process
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
125.6 °F - closed cup
flash_point_c
52 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A practical chemoenzymatic synthesis of a key intermediate of antifungal agents.
Yasohara Y, et al.
Tetrahedron Letters, 42(19), 3331-3333 (2001)
Regioselective ortho-lithiation of chloro and bromo substituted fluoroarenes.
Mongin F and Schlosser M.
Tetrahedron Letters, 37(36), 6551-6554 (1996)
Ram Shankar Upadhayaya et al.
Bioorganic & medicinal chemistry, 12(9), 2225-2238 (2004-04-15)
A series of (2R,3S)-2-(2,4-difluorophenyl)-3-(5-[2-[4-aryl-piperazin-1-yl]-ethyl]-tetrazol-2-yl)-1-[1,2,4]-triazol-1-yl-butan-2-ol (11a-n) and (2R,3S)-2-(2,4-difluorophenyl)-3-(5-[2-[4-aryl-piperazin-1-yl]-ethyl]-tetrazole-1-yl)-1-[1,2,4]-triazol-1-yl-butan-2-ol (12a-n) has been synthesized. The antifungal activity of compounds was evaluated by in vitro agar diffusion and broth dilution assay. Compounds 11d and its positional isomer 12d having 3-trifluoromethyl substitution on the phenyl
Ji Gwang Yu et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 23(63), 16044-16050 (2017-08-24)
Four dibenzofuran-type host materials substituted with a carbazolylcarbazole moiety were synthesized to investigate the effect of substitution position on the material parameters and device performances of host materials. The carbazolylcarbazole moiety was substituted at the 1-, 2-, 3-, and 4-positions
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service