261394
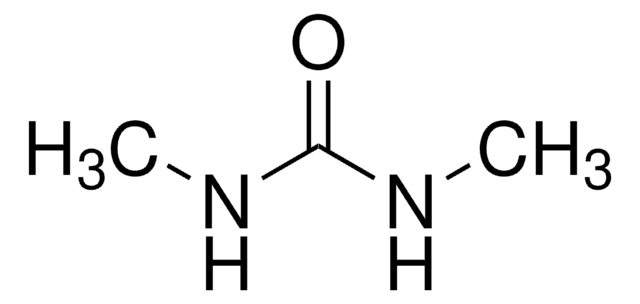
1,1-Dimethylurea
99%
Synonym(s):
N,N-Dimethylurea
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3)2NCONH2
CAS Number:
Molecular Weight:
88.11
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
99%
form
solid
mp
178-183 °C (lit.)
solubility
water: soluble 5%, clear, colorless
functional group
amine
SMILES string
CN(C)C(N)=O
InChI
1S/C3H8N2O/c1-5(2)3(4)6/h1-2H3,(H2,4,6)
InChI key
YBBLOADPFWKNGS-UHFFFAOYSA-N
Gene Information
human ... EPHX2(2053)
mouse ... Ephx2(13850)
General description
Nonlinear optical properties of 1,1-dimethylurea (N,N′ dimethylurea), have been evaluated through second-harmonic generation.
Application
1,1-Dimethylurea (N,N-dimethylurea) has been used in the Dowex-50W ion exchange resin-promoted synthesis of N,N′-disubstituted-4-aryl-3,4-dihydropyrimidinones.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Frank F Millenaar et al.
The New phytologist, 184(1), 141-152 (2009-06-30)
Environmental challenges such as low light intensity induce differential growth-driven upward leaf movement (hyponastic growth) in Arabidopsis thaliana. However, little is known about the physiological regulation of this response. Here, we studied how low light intensity is perceived and translated
S Sandler et al.
Acta pharmacologica et toxicologica, 53(5), 392-400 (1983-11-01)
The possible protective effects in vitro of the hydroxyl radical scavenger dimethyl urea (6 mg/ml) and the poly(ADP-ribose)synthetase inhibitors theophylline (5 mM) and nicotinamide (0.75 mg/ml) against streptozotocin (SZ) induced deterioration of islet metabolism were investigated using isolated mouse pancreatic
W J Caspary et al.
Mutation research, 174(4), 285-293 (1986-08-01)
Methylisocyanate (MIC) induced mutagenic responses in the absence of exogenous activation in the mouse lymphoma cell forward mutation assay at concentrations as low as 8-24 microM. MIC produced predominantly small mutant colonies, suggesting the possibility of clastogenic activity. The intermediate
G L Wilson et al.
Diabetologia, 27(6), 587-591 (1984-12-01)
In studies to evaluate possible inhibitors of the B-cell toxin, streptozotocin, the superoxide scavenger, superoxide dismutase, did not prevent or reduce the toxic effects of streptozotocin as determined by loss of insulin secretion from rat pancreatic B cells in monolayer
S Sandler et al.
Diabetologia, 23(4), 374-378 (1982-10-01)
The protective effect on streptozotocin-induced diabetes of dimethyl urea, a hydroxyl radical scavenger, has been evaluated in vivo and in vitro. Pretreatment with dimethyl urea before a single diabetogenic dose of streptozotocin partially protected NMRI mice from hyperglycaemia, whereas the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service