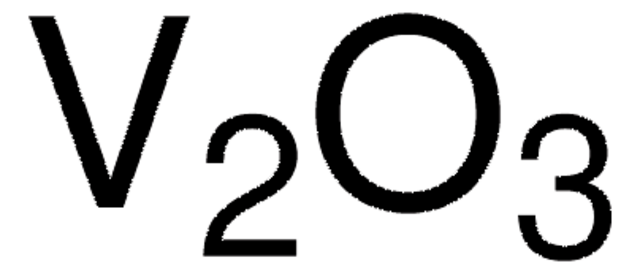This material particle size will be 850 um or less.
223794
Vanadium(V) oxide
≥98%
Synonym(s):
Divanadium pentaoxide, Divanadium pentoxide, Pentaoxodivanadium, Vandia
Select a Size
About This Item
Recommended Products
Quality Level
assay
≥98%
form
powder
reaction suitability
core: vanadium
reagent type: catalyst
mp
690 °C (lit.)
density
3.35 g/mL at 25 °C (lit.)
SMILES string
O=[V](=O)O[V](=O)=O
InChI
1S/5O.2V
InChI key
GNTDGMZSJNCJKK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
application
signalword
Danger
Hazard Classifications
Acute Tox. 2 Inhalation - Acute Tox. 3 Oral - Aquatic Chronic 2 - Carc. 1B - Lact. - Muta. 2 - Repr. 2 - STOT RE 1 Inhalation - STOT SE 3
target_organs
Respiratory system, Respiratory Tract
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Professor Chen (Nankai University, China) and his team explain the strategies behind their recent record-breaking organic solar cells, reaching a power conversion efficiency of 17.3%.
-
What is the particle size of the 223794-100G Vanadium Oxide?
1 answer-
Helpful?
-
-
What is the Department of Transportation shipping information for this product?
1 answer-
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Helpful?
-
-
What is the density of Product 223794, Vanadium(V) oxide?
1 answer-
The reported density is 3.35 g/mL at 25°C(lit.)
Helpful?
-
-
What is Product 223794, Vanadium(V) oxide, soluble in?
1 answer-
According to the chemicals encyclopedia published by the Royal Society of Chemistry (ed. 13, 9987), one gram dissolves in approximately 125 mL of water. It is soluble in concentrated acids, forming red to yellow solutions; soluble in alkalies, forming vanadates; it is insoluble in alcohol.
Helpful?
-
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service