D2322
Diaphorase from Clostridium kluyveri
recombinant, expressed in E. coli, Animal-component free
Synonym(s):
Diaphorase, Lipoamide Dehydrogenase, Lipoyl Dehydrogenase
About This Item
Recommended Products
recombinant
expressed in E. coli
description
Histidine-Tagged
form
solid
specific activity
>=30 units/mg protein (biuret)
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Application
Biochem/physiol Actions
Other Notes
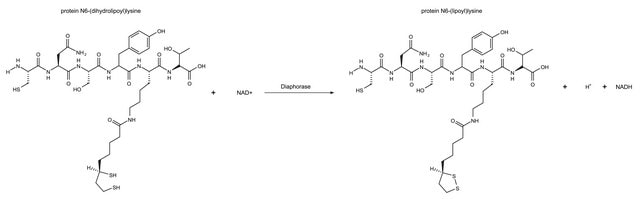
Diaphorases which are specific for either β-NADH or β-NADPH are known. The pig heart enzyme of Straub seems to have native diaphorase (β-NADH specific) as well as lipoic and lipoamide dehydrogenase activities. It is reported to be a single protein. However, Massey reports that "diaphorase" is probably a denatured lipoamide dehydrogenase. Pre-incubation of the pig heart preparation with Cu2+ reduces the lipoamide dehydrogenase activity and proportionately increases the β-NADH diaphorase activity. In our laboratory, we have demonstrated this copper effect to some degree on the pig heart enzyme, but no appreciable effect was observed on the Clostridium kluyveri or torula yeast preparations. The lipoamide dehydrogenase:diaphorase ratio is a measure of the denaturation.
Unit Definition
Physical form
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service