459542
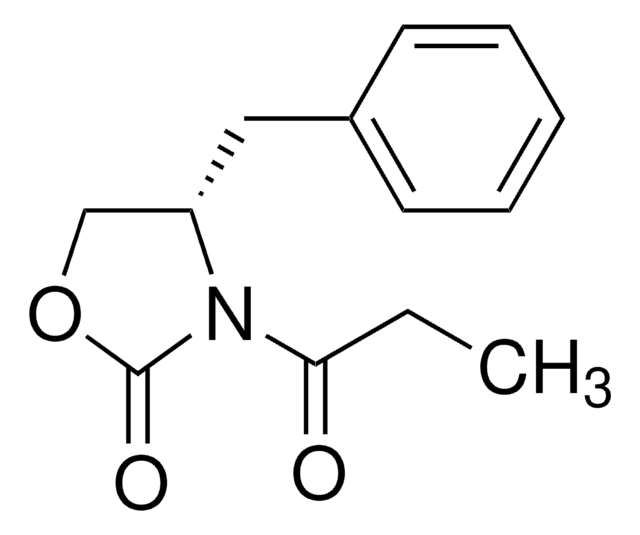
(R)-(−)-4-Benzyl-3-propionyl-2-oxazolidinone
99%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C13H15NO3
CAS Number:
Molecular Weight:
233.26
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
99%
form
solid
optical activity
[α]20/D −102°, c = 1 in ethanol
optical purity
ee: 99% (HPLC)
mp
44-46 °C (lit.)
SMILES string
CCC(=O)N1[C@@H](COC1=O)Cc2ccccc2
InChI
1S/C13H15NO3/c1-2-12(15)14-11(9-17-13(14)16)8-10-6-4-3-5-7-10/h3-7,11H,2,8-9H2,1H3/t11-/m1/s1
InChI key
WHOBYFHKONUTMW-LLVKDONJSA-N
General description
(R)-(−)-4-Benzyl-3-propionyl-2-oxazolidinone is used as a building block in organic synthesis for the preparation of oxazolidinone derivatives.
Application
(R)-(−)-4-Benzyl-3-propionyl-2-oxazolidinone can be used as a building block for the preparation of methyl 3-[(S)-3-((R)-4-benzyl-2-oxooxazolidin-3-yl)-2-methyl-3-oxopropyl]benzoate by treating with strong base followed by the addition of methyl 3-bromomethyl benzoate.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Oxazolidinone cross-alkylation during Evans? asymmetric alkylation reaction
Fresno N, et al.
Tetrahedron, 67(47), 9104-9111 (2011)
A Synthetic Route to β-Hydroxytyrosine-Derived Tetramic Acids: Total Synthesis of the Fungal Metabolite F-14329
Bruckner, S, et al.
Chemistry?A European Journal , 23(24), 5692-5695 (2017)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service