All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H11NS2
CAS Number:
Molecular Weight:
161.29
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
≥98.0%
form
solid
optical activity
[α]20/D -37±3°, c = 1 in chloroform
optical purity
ee: ≥99:1 (LC)
mp
69-71 °C
SMILES string
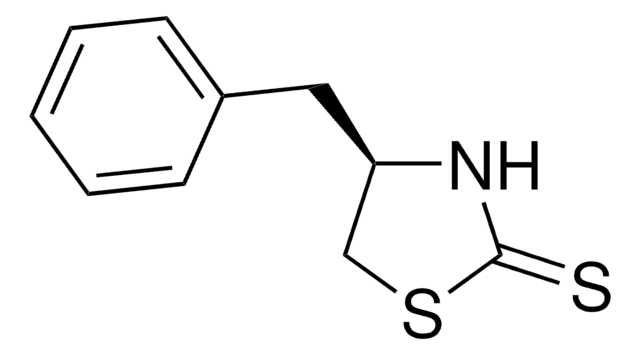
CC(C)[C@H]1CSC(=S)N1
InChI
1S/C6H11NS2/c1-4(2)5-3-9-6(8)7-5/h4-5H,3H2,1-2H3,(H,7,8)/t5-/m1/s1
InChI key
CWIZUGZKLJDJLE-RXMQYKEDSA-N
Application
(S)-4-Isopropylthiazolidine-2-thione may be used as a chiral auxiliary for the stereochemical induction at the phosphorus atom during the diastereoselective synthesis of aryloxy phosphoramidate prodrugs of 3′-deoxy-2′,3′-didehydrothymidine monophosphate (d4TMP).
A highly selective and efficient chiral auxiliary which can be directly reduced to its corresponding aldehyde and the chiral auxiliary by reductive cleavage with diisobutylaluminum hydride.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Diastereoselective synthesis of aryloxy phosphoramidate prodrugs of 3'-deoxy-2', 3'-didehydrothymidine monophosphate.
Roman CA, et al.
Journal of Medicinal Chemistry, 53(21), 7675-7681 (2010)
Velazquez. F.; Olivo, H. F.
Current Organic Chemistry, 6, 303-303 (2002)
M T Crimmins et al.
Organic letters, 2(6), 775-777 (2001-02-07)
[formula: see text] Asymmetric aldol additions using chlorotitanium enolates of thiazolidinethione propionates proceed with high diastereoselectivity for the "Evans" or "non-Evans" syn product depending on the nature and amount of the base used. With (-)-sparteine as the base, selectivities of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service