791056P
Avanti
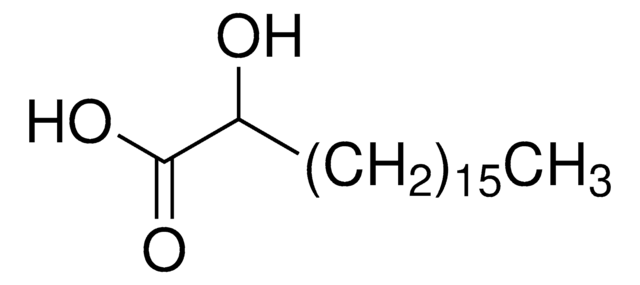
2-OHOA
2-hydroxyoleic acid (sodium salt), powder
Synonym(s):
Minerval; NaCHOleate; 2OHOA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C18H33NaO3
CAS Number:
Molecular Weight:
320.44
UNSPSC Code:
12352211
NACRES:
NA.25
Recommended Products
Assay
>99% (TLC)
form
powder
packaging
pkg of 1 × 1 g (791056P-1g)
manufacturer/tradename
Avanti Research™ - A Croda Brand 791056P
lipid type
neutral lipids
neutral glycerides
shipped in
dry ice
storage temp.
−20°C
General description
2-hydroxyoleic acid (2-OHOA) is a synthetic and non-β oxidation-metabolizable oleic acid derivative. It contains 18 carbon atoms in its fatty acid chain length.
Application
2-hydroxyoleic acid (2-OHOA) is suitable for use:
- to study its antihypertensive action in adult spontaneously hypertensive rats (SHRs)
- to study the chemical structure-effect relation of 2-OHOA in the reduction of body weight in rats
- as an antitumor compound, to study its effect on xenografts lipidome based on imaging mass spectrometry (IMS)
Biochem/physiol Actions
2-Hydroxy Oleic Acid is an inducer of cell cycle arrest and apoptosis in several cancer cell lines, including glioma, leukemia, breast and colon cancer lines. 2-Hydroxy Oleic Acid increases sphingomyelin (SM) levels in the membranes of tumor cells, which typically display decreased SM membrane content, and remodeled membranes, compared with normal cells. The compound has no effect on SM levels in non-cancer cells.
2-hydroxyoleic acid (2-OHOA) acts as a potential antihypertensive agent. Oral administration of 2-OHOA is used to reduce body weight.
Packaging
20 mL Clear Glass Screw Cap Vial (791056P-1g)
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Structure-effect relation of C18 long-chain fatty acids in the reduction of body weight in rats
Vogler O, et al.
International Journal of Obesity, 32(3), 464-464 (2008)
Victoria Lladó et al.
Biochimica et biophysica acta, 1838(6), 1619-1627 (2014-02-15)
This review summarizes the cellular bases of the effects of NaCHOleate (2-hydroxyoleic acid; 2OHOA; Minerval) against glioma and other types of tumors. NaCHOleate, activates sphingomyelin synthase (SGMS) increasing the levels of cell membrane sphingomyelin (SM) and diacylglycerol (DAG) together with
Victoria Llado et al.
Journal of cellular and molecular medicine, 14(3), 659-670 (2009-05-06)
Minerval is an oleic acid synthetic analogue that impairs lung cancer (A549) cell proliferation upon modulation of the plasma membrane lipid structure and subsequent regulation of protein kinase C localization and activity. However, this mechanism does not fully explain the
Alena Khmelinskaia et al.
Langmuir : the ACS journal of surfaces and colloids, 30(8), 2117-2128 (2014-02-05)
Recent research regarding 2-hydroxylated fatty acids (2OHFAs) showed clear evidence of their benefits in the treatment of cancer, inflammation, and neurodegenerative disorders such as Alzheimer's disease. Monolayer compressibility isotherms and isothermal titration calorimetry of 2OHFA (C18-C22) in phosphatidylcholine/phosphatidylethanolamine/sphingomyelin/cholesterol (1:1:1:1 mole
Silvia Terés et al.
Proceedings of the National Academy of Sciences of the United States of America, 109(22), 8489-8494 (2012-05-16)
Despite recent advances in the development of new cancer therapies, the treatment options for glioma remain limited, and the survival rate of patients has changed little over the past three decades. Here, we show that 2-hydroxyoleic acid (2OHOA) induces differentiation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service