754080
ADT
sublimed, 97%
Synonym(s):
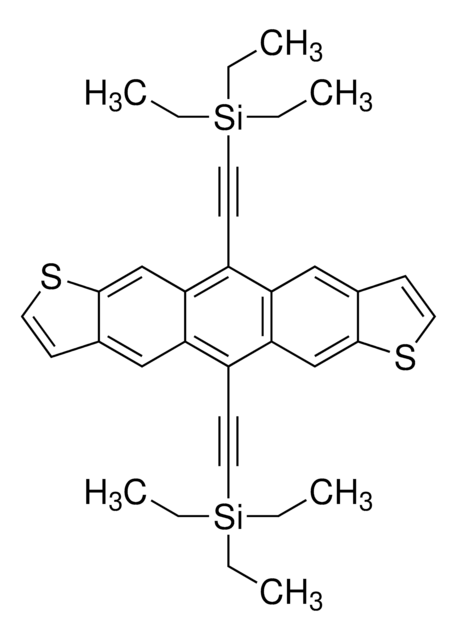
Anthra[2,3-b:6,7-b′]dithiophene
About This Item
Recommended Products
Assay
97%
form
sublimed
loss
0.5 wt. %, 280 °C
semiconductor properties
P-type (mobility=0.3 cm2/V·s)
SMILES string
c1cc2cc3cc4cc5sccc5cc4cc3cc2s1
InChI
1S/C18H10S2/c1-3-19-17-9-15-8-14-6-12-2-4-20-18(12)10-16(14)7-13(15)5-11(1)17/h1-10H
InChI key
DAMUWSYTQPWFIY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Organic materials in optoelectronic devices like LEDs and solar cells are of significant academic and commercial interest.
Intrinsically stretchable active layers for organic field-effect transistors (OFET) are discussed. Polymer structural modification & post-polymerization modifications are 2 methods to achieve this.
Solution-processed organic photovoltaic devices (OPVs) have emerged as a promising clean energy generating technology due to their ease of fabrication, potential to enable low-cost manufacturing via printing or coating techniques, and ability to be incorporated onto light weight, flexible substrates.
Thin, lightweight, and flexible electronic devices meet widespread demand for scalable, portable, and robust technology.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![Dinaphtho[2,3-b:2′,3′-f]thieno[3,2-b]thiophene sublimed grade, 99%](/deepweb/assets/sigmaaldrich/product/structures/196/451/8a650b8e-abbb-4ef1-9be4-73f223165062/640/8a650b8e-abbb-4ef1-9be4-73f223165062.png)



![Thieno[3,2-b]thiophene 95%](/deepweb/assets/sigmaaldrich/product/structures/353/609/429fd4bf-e217-4371-80a3-9e5a4d88908b/640/429fd4bf-e217-4371-80a3-9e5a4d88908b.png)




