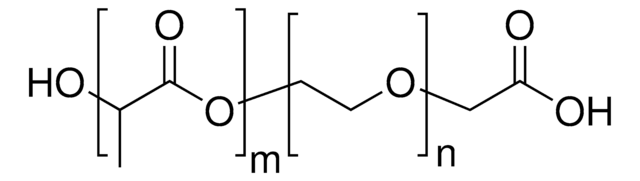
Unfortunately, the number of repeat units is not determined. The inherent and reduced viscosities are 0.512 and 0.526 dL/g, respectively. The viscosities are measured by dissolving the product in concentrated sulfuric acid to form a solution with a concentration of 0.0998 g/dL at 25°C.
667846
BBL
Synonym(s):
Poly(benzimidazobenzophenanthroline)
Select a Size
Select a Size
About This Item
Recommended Products
description
Band gap: 1.9 eV
Quality Level
form
solid
Orbital energy
HOMO -5.9 eV
LUMO -4.0 eV
OPV Device Performance
ITO/MEH-PPV/BBL/Al
- Short-circuit current density (Jsc): 1.98 mA/cm2
- Open-circuit voltage (Voc): 0.93 V
- Fill Factor (FF): 0.47
- Power Conversion Efficiency (PCE): 1.1 %
ITO/PPV/BBL/Al
- Short-circuit current density (Jsc): 2.15 mA/cm2
- Open-circuit voltage (Voc): 1.1 V
- Fill Factor (FF): 0.5
- Power Conversion Efficiency (PCE): 1.5 %
semiconductor properties
N-type (mobility=0.1 cm2/V·s)
P-type (mobility=0.4 cm2/V·s)
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
The development of high-performance conjugated organic molecules and polymers has received widespread attention in industrial and academic research.
Intrinsically stretchable active layers for organic field-effect transistors (OFET) are discussed. Polymer structural modification & post-polymerization modifications are 2 methods to achieve this.
Fabrication procedure of organic field effect transistor device using a soluble pentacene precursor.
Thin, lightweight, and flexible electronic devices meet widespread demand for scalable, portable, and robust technology.
-
Hello, What is the number of repeat unit or viscosity of this polymer?
1 answer-
Helpful?
-
-
Is there any molecular weight information available for the BBL polymer (item # 667846)?
1 answer-
The molecular weight for this product is not determined. However, historical data suggest that it is 304.2 g/mol.
Helpful?
-
-
Hello, what is the viscosity (or number of repeat units) of this polymer?
1 answer-
The viscosity of this material is not tested on a lot-to-lot basis. However, historical data indicates an intrinsic viscosity of approximately 1.6 dl/g in Methanesulfonic acid at 25°C.
Helpful?
-
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![Naphtho[1,2-c:5,6-c′]bis[1,2,5]thiadiazole-5,10-diboronic acid bis(pinacol) ester 95%](/deepweb/assets/sigmaaldrich/product/structures/396/334/bb0914db-5c9a-4565-ba76-30dd4bc4ba87/640/bb0914db-5c9a-4565-ba76-30dd4bc4ba87.png)