W291102
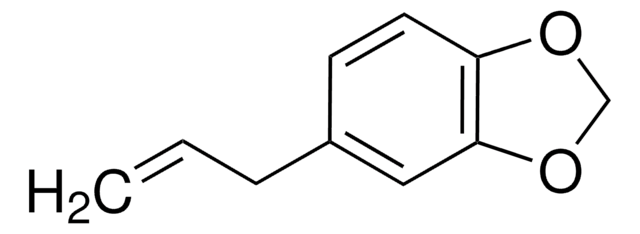
Piperonal
≥99%, FCC, FG
Synonyme(s) :
1,3-Benzodioxole-5-carboxaldehyde, 3,4-(Methylenedioxy)benzaldehyde, Heliotropin
About This Item
Produits recommandés
Source biologique
synthetic
Niveau de qualité
Qualité
FG
Fragrance grade
Halal
Kosher
Agence
follows IFRA guidelines
meets purity specifications of JECFA
Conformité réglementaire
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FCC
FDA 21 CFR 182.60
Pression de vapeur
1 mmHg ( 87 °C)
Pureté
≥99%
Point d'ébullition
264 °C (lit.)
Pf
35-39 °C (lit.)
Application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
Allergène alimentaire
no known allergens
Allergène de parfum
heliotropine
Propriétés organoleptiques
cherry; sweet; vanilla
Chaîne SMILES
[H]C(=O)c1ccc2OCOc2c1
InChI
1S/C8H6O3/c9-4-6-1-2-7-8(3-6)11-5-10-7/h1-4H,5H2
Clé InChI
SATCULPHIDQDRE-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Application
- The synthesis and characterisation of MDMA derived from a catalytic oxidation of material isolated from black pepper reveals potential route specific impurities.: This study explores the synthesis and characterization of MDMA from piperonal, highlighting potential impurities unique to this synthesis route. This research has implications for forensic science and the identification of synthetic routes for MDMA (Plummer et al., 2016).
- Design, synthesis, and biological evaluation of platensimycin analogues with varying degrees of molecular complexity.: This paper details the synthesis of platensimycin analogues using piperonal derivatives. The study evaluates the biological activities of these analogues, contributing to the development of new antibacterial agents (Nicolaou et al., 2008).
- Synthesis and use of 4-peptidylhydrazido-N-hexyl-1,8-naphthalimides as fluorogenic histochemical substrates for dipeptidyl peptidase IV and tripeptidyl peptidase I.: This research presents the synthesis of piperonal-based substrates for histochemical applications, enabling the study of enzyme activities in biochemical assays (Ivanov et al., 2009).
Actions biochimiques/physiologiques
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Skin Sens. 1
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
150.1 °F
Point d'éclair (°C)
65.62 °C
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique