921726
Bis-Diazirine polymer crosslinker
Synonyme(s) :
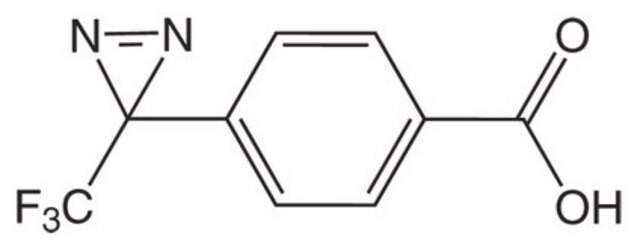
3,3′-((Perfluoropropane-2,2-diyl)bis(4,1-phenylene))bis(3-(trifluoromethyl)-3H-diazirine), BondLynx Gen-I
About This Item
Produits recommandés
Température de stockage
2-8°C
Niveau de qualité
Application
Our bis-diazirine molecule allow remarkably efficient and stable perovskite solar cells. A study showed it contribution to retaining nearly 99% of their initial efficiency even after 1,000 hours of continuous illumination. At constant heat (60°C) bis-diazirine treated PSCs maintained nearly 98% efficiency after 600 hours of continuous operation, whilst untreated lost 27% efficiency under the same conditions. Overall, bis-diazirine treated PSCs achieved a high certified efficiency of over 24% with long operational stability over 1,000 hours.
Further, it can be used in adhesive and textile strengthening applications. Diazirine crosslinkers don’t distinguish between polymer substrates and can be used to bond dissimilar materials like PP and PE together into rigid thermoset plastics. It has been demonstrated to bond polypropylene, high density polyethylene (HDPE), and other polymers to each other, and to strengthen ultrahigh molecular weight polyethylene (UHMWPE) textiles by crosslinking between individual fibers. Bis-diazirines may also have utility in tissue bonding applications.
Attention
Yoshida correlations and mechanical testing indicate that the product is not a likely explosion hazard, but all diazirines should be handled as potential shock-sensitive materials.
Autres remarques
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Org. Perox. C
Code de la classe de stockage
5.2 - Organic peroxides and self-reacting hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique