All Photos(1)
About This Item
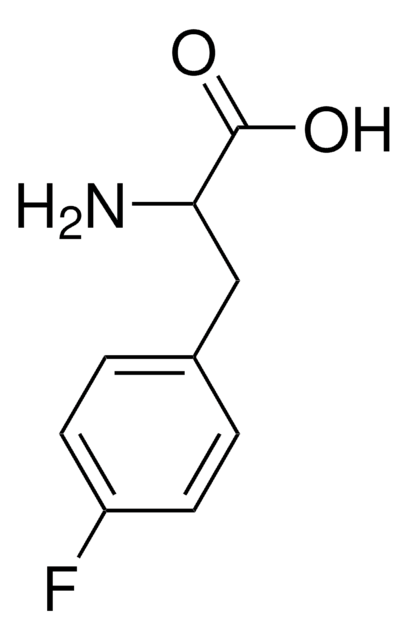
Linear Formula:
FC6H4CH2CH(NH2)COOH
CAS Number:
Molecular Weight:
183.18
Beilstein:
3201186
EC Number:
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥95% (NT)
≥98% (HPLC)
≥98%
form
powder
reaction suitability
reaction type: solution phase peptide synthesis
color
white to faint brown
mp
243-246 °C (lit.)
application(s)
cell analysis
peptide synthesis
SMILES string
NC(Cc1ccccc1F)C(O)=O
InChI
1S/C9H10FNO2/c10-7-4-2-1-3-6(7)5-8(11)9(12)13/h1-4,8H,5,11H2,(H,12,13)
InChI key
NYCRCTMDYITATC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reactant involved in:
- Studying mechanism of P 450-mediated oxidation and rearrangement
- Conversion of racemic α-arylalanines to (R)-β-arylalanines
- Ribosomal translation of unnatural peptides
- Synthesis of diisopropylpyridine acetamides for use as Kv1.5 potassium channel antagonists
- Enantioselective hydrolysis of esters for resolution of nonprotein amino acids
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Johnny Castillo Meleán et al.
Organic & biomolecular chemistry, 9(3), 765-769 (2010-11-23)
2-[(18)F]Fluoro-L-phenylalanine and 2-[(18)F]fluoro-L-tyrosine have been developed as promising radiopharmaceuticals for molecular imaging using positron emission tomography (PET). However, the lack of a convenient radiosynthetic pathway has limited their practical use. In this work a new three-step nucleophilic synthesis of these
K Kubota et al.
Journal of nuclear medicine : official publication, Society of Nuclear Medicine, 37(2), 320-325 (1996-02-01)
L-[methyl-11C]methionine (11C-Met) is a useful tracer for tumor imaging with PET. The drawbacks include a short half-life and high physiological accumulation in abdominal organs. To overcome these shortfalls, the feasible use of [18F]fluorophenylalanine (18F-Phe), which shares the same amino acid
Kensuke Okuda et al.
Biochemistry, 44(17), 6675-6684 (2005-04-27)
The ribosome-catalyzed peptidyl transferase reaction displays a complex pH profile resulting from two functional groups whose deprotonation is important for the reaction, one within the A-site substrate and a second unidentified group thought to reside in the rRNA peptidyl transferase
T T Otani et al.
Journal of pharmaceutical sciences, 71(2), 214-216 (1982-02-01)
Twelve derivatives of 0-fluoro-dl-phenylalanine containing fluorine, chlorine, methoxy, and nitro radicals in various positions of the aromatic ring of the benzoyl group were prepared and tested in a Lactobacillus casei system. It was found that most substitutions in the benzoyl
H Nakamichi et al.
Nuclear medicine and biology, 21(7), 959-962 (1994-10-01)
The anabolism of isotopically labeled amino acids was compared between the cerebrum and the cerebellum in conscious rat at three feeding conditions. After L-[2-18F]fluorophenylalanine and L-[2,6-3H]phenylalanine injections, the incorporation rate of both radioactivity into protein fraction showed no difference between
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service