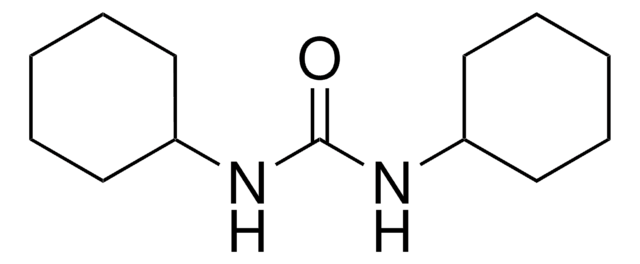
36650
DCC
≥99.0% (GC), for peptide synthesis
Sinónimos:
N,N′-Dicyclohexylcarbodiimide
About This Item
Productos recomendados
product name
DCC, puriss., ≥99.0% (GC)
grade
puriss.
Quality Level
assay
≥99.0% (GC)
form
solid
reaction suitability
reaction type: Coupling Reactions
bp
122-124 °C/6 mmHg (lit.)
mp
32.0-37.0 °C
34-35 °C (lit.)
solubility
methylene chloride: 0.1 g/mL, clear, colorless
application(s)
peptide synthesis
SMILES string
C1CCC(CC1)N=C=NC2CCCCC2
InChI
1S/C13H22N2/c1-3-7-12(8-4-1)14-11-15-13-9-5-2-6-10-13/h12-13H,1-10H2
InChI key
QOSSAOTZNIDXMA-UHFFFAOYSA-N
Gene Information
human ... EPHX2(2053)
mouse ... Ephx2(13850)
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
It may be also used to synthesize:
- 1,3-Thiazetedine derivatives via [2+2] cycloaddition with 2-phenylethenyl- and 2-(4-nitrophenyl)ethenyl isothiocyanates.
- 1,3,5-Oxadiazine-4-thiones via [4+2] cycloaddition with benzoyl isothiocyanates.
- Sterically hindered 1,3,4-oxadiazole derivatives by reacting with (N-isocyanimino)triphenylphosphorane the presence of aromatic (or heteroaromatic) carboxylic acids.
Other Notes
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Sens. 1
Storage Class
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
wgk_germany
WGK 3
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico