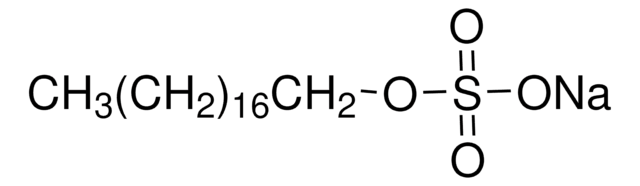318183
Methyl sulfate sodium salt
Sinónimos:
Monomethyl ester sulfuric acid sodium salt, Sodium methyl sulfate
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
CH3OSO3Na
Número de CAS:
Peso molecular:
134.09
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
form
powder
Quality Level
impurities
<3% methanol
<5% water
mp
210 °C (lit.)
SMILES string
[Na+].COS([O-])(=O)=O
InChI
1S/CH4O4S.Na/c1-5-6(2,3)4;/h1H3,(H,2,3,4);/q;+1/p-1
InChI key
DZXBHDRHRFLQCJ-UHFFFAOYSA-M
Application
Methyl sulfate sodium salt can be used to synthesize:
- Methylsulfate anion based 1,3,4-trialkyl-1,2,3-triazolium ionic liquids for use in Morita–Baylis–Hillman reaction.
- Anisole by reacting with phenol.
- Hydroxyanilino quinolines for use as RET kinase inhibitors.
Reactant or reagent involved in:
- Studying lamellar structure formation in hybrid nanomaterials created by miniemulsion
- EPR studies of radical ions radiolytically generated from ionic liquids, used as a reference
- Micellular studies specifically the rate-retarding effects on hydrolysis of substituted 1-benzoyl-1,2,4-triazoles and self-assembly / microstructure of mixed micelles
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
The discovery of substituted 4-(3-hydroxyanilino)-quinolines as potent RET kinase inhibitors
Robinett R G, et al.
Bioorganic & Medicinal Chemistry Letters, 17(21), 5886-5893 (2007)
Synthesis of anisole from phenol and sodium methyl sulfate, a byproduct from synthesis of medicine intermediates
Guoping W, et al.
Petrochemical Technology, 45(11), 1337-1337 (2016)
Synthesis of new triazolium-based ionic liquids and their use in the Morita-Baylis-Hillman reaction
F Alioune, et al.
Tetrahedron Letters, 56(36), 5128-5131 (2015)
Antonio Arroyo et al.
The Journal of biological chemistry, 277(51), 49965-49975 (2002-10-12)
Resolution of inflammation requires clearance of activated neutrophils by phagocytes in a manner that protects adjacent tissues from injury. Mechanisms governing apoptosis and clearance of activated neutrophils from inflamed areas are still poorly understood. We used dimethylsulfoxide-differentiated HL-60 cells showing
Bindhu V Karanam et al.
Drug metabolism and disposition: the biological fate of chemicals, 32(10), 1061-1068 (2004-07-02)
MK-0767 [(+/-)-5-[(2,4-dioxothiazolidin-5-yl)methyl]-2-methoxy-N-[[(4-trifluoromethyl)phenyl]methyl]benzamide], a thiazolidinedione (TZD)-containing peroxisome proliferator-activated receptor agonist, is a rapidly interconverting racemate that possesses a chiral center at the five position of the TZD ring. M25 is a methyl sulfide metabolite generated from MK-0767 following CYP3A4-mediated TZD ring
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico