292710
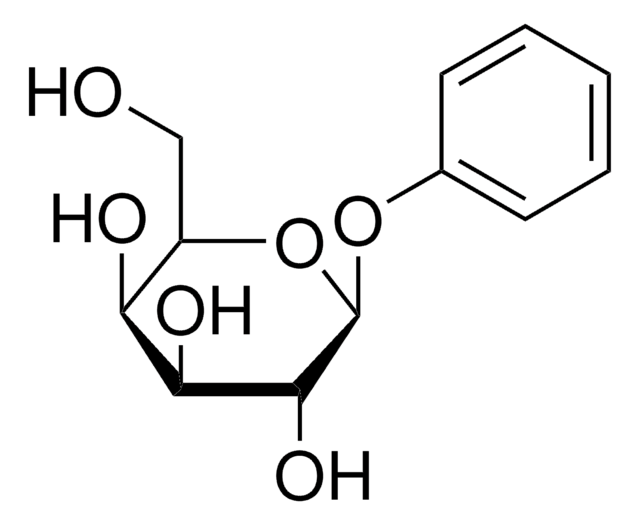
Phenyl β-D-glucopyranoside
≥95.0%
Synonym(s):
Phenyl beta-D-glucoside
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C12H16O6
CAS Number:
Molecular Weight:
256.25
Beilstein:
87517
EC Number:
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥95.0%
form
powder
optical activity
[α]25/D −70°, c = 1 in H2O
mp
176-178 °C (lit.)
SMILES string
OC[C@H]1O[C@@H](Oc2ccccc2)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C12H16O6/c13-6-8-9(14)10(15)11(16)12(18-8)17-7-4-2-1-3-5-7/h1-5,8-16H,6H2/t8-,9-,10+,11-,12-/m1/s1
InChI key
NEZJDVYDSZTRFS-RMPHRYRLSA-N
Application
Phenyl β-D-glucopyranoside can be used:
- As a starting material for the synthesis of various derivatives of β-D-glucopyranosides with potential application as anti-HIV agents.
- As a model for glycosides in the gas phase for their spectroscopic investigation.
- As an internal standard in GC and GC-MS quantitative analyses.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
4-(Arylamino)phenyl alpha-D-glucopyranosides as potential anti-HIV agents.
J C Briggs et al.
Carbohydrate research, 282(2), 293-298 (1996-03-18)
Sugars in the gas phase: the spectroscopy and structure of jet-cooled phenyl ?-D-glucopyranoside.
Talbot FO and Simons JP
Physical Chemistry Chemical Physics, 4(15), 3562-3565 (2002)
Anke Reinders et al.
Plant, cell & environment, 29(10), 1871-1880 (2006-08-26)
Plant sucrose transporters (SUTs) are members of the glycoside-pentoside-hexuronide (GPH) cation symporter family (TC2.A.2) that is part of the major facilitator superfamily (MFS). All plant SUTs characterized to date function as proton-coupled symporters and catalyze the cellular uptake of sucrose.
Chunyan Bao et al.
Carbohydrate research, 339(7), 1311-1316 (2004-04-29)
A new hydrogel based on a substituted phenyl glucoside with a Schiff base in the aglycon was synthesized, and the self-assembling characteristics was studied. FTIR spectra, UV-vis absorption spectra and X-ray diffraction (XRD) revealed that pi-pi interactions between the Schiff
Ying Na et al.
Bioorganic chemistry, 39(3), 111-113 (2011-03-26)
The spontaneous hydrolysis of glycosylamines, where the aglycone is either a primary amine or ammonia, is over a hundred million-times faster than that of O- or S-glycosides. The reason for this (as pointed out by Capon and Connett in 1965)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service