B4380
Bromobimane
≥97% purity, powder
Sinónimos:
Monobromobimane
About This Item
Productos recomendados
product name
Bromobimane, ≥97% (HPLC)
Quality Level
assay
≥97% (HPLC)
form
powder
color
yellow
mp
161 °C
solubility
acetonitrile: 20 mg/mL
ε (extinction coefficient)
4.6-5.1 at 396-398 nm in H2O
application(s)
diagnostic assay manufacturing
hematology
histology
storage temp.
−20°C
SMILES string
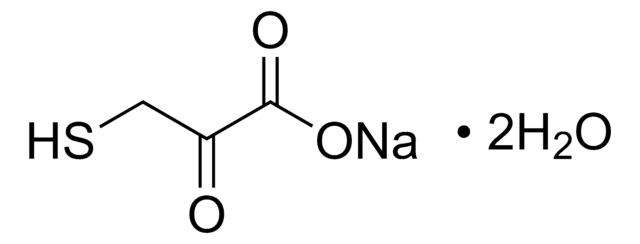
CC1=C(C)C(=O)N2N1C(CBr)=C(C)C2=O
InChI
1S/C10H11BrN2O2/c1-5-7(3)12-8(4-11)6(2)10(15)13(12)9(5)14/h4H2,1-3H3
InChI key
AHEWZZJEDQVLOP-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
Bromobimane has been used for the quantitative measurement of free hydrogen sulfide in vivo and in vitro. It has been used for the labeling of proteins containing thiol groups.
Biochem/physiol Actions
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Certificados de análisis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico